[English] 日本語
 Yorodumi
Yorodumi- PDB-4keb: Human dihydrofolate reductase complexed with NADPH and 5-{3-[3-me... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4keb | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human dihydrofolate reductase complexed with NADPH and 5-{3-[3-methoxy-5-(isoquin-5-yl)phenyl]but-1-yn-1-yl}6-ethylpyrimidine-2,4-diamine | ||||||
 Components Components | Dihydrofolate reductase | ||||||
 Keywords Keywords | OXIDOREDUCTASE/OXIDOREDUCTASE INHIBITOR / Oxidoreductase / 5 / 6 / 7 / 8-tetrahydrofolate / NADP+ / hydride shift / cytosol / OXIDOREDUCTASE-OXIDOREDUCTASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of removal of superoxide radicals / tetrahydrobiopterin biosynthetic process / Metabolism of folate and pterines / tetrahydrofolate metabolic process / response to methotrexate / sequence-specific mRNA binding / folic acid binding / axon regeneration / dihydrofolate metabolic process / G1/S-Specific Transcription ...regulation of removal of superoxide radicals / tetrahydrobiopterin biosynthetic process / Metabolism of folate and pterines / tetrahydrofolate metabolic process / response to methotrexate / sequence-specific mRNA binding / folic acid binding / axon regeneration / dihydrofolate metabolic process / G1/S-Specific Transcription / dihydrofolate reductase / dihydrofolate reductase activity / folic acid metabolic process / tetrahydrofolate biosynthetic process / NADPH binding / Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation / one-carbon metabolic process / mRNA regulatory element binding translation repressor activity / NADP binding / negative regulation of translation / mRNA binding / mitochondrion / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.45 Å MOLECULAR REPLACEMENT / Resolution: 1.45 Å | ||||||
 Authors Authors | Lamb, K.M. / Anderson, A.C. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2013 Journal: Biochemistry / Year: 2013Title: Elucidating features that drive the design of selective antifolates using crystal structures of human dihydrofolate reductase. Authors: Lamb, K.M. / G-Dayanandan, N. / Wright, D.L. / Anderson, A.C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4keb.cif.gz 4keb.cif.gz | 195.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4keb.ent.gz pdb4keb.ent.gz | 156.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4keb.json.gz 4keb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ke/4keb https://data.pdbj.org/pub/pdb/validation_reports/ke/4keb ftp://data.pdbj.org/pub/pdb/validation_reports/ke/4keb ftp://data.pdbj.org/pub/pdb/validation_reports/ke/4keb | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4kakC  4kbnC  4kd7C  4kfjC  2c2sS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 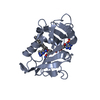
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 21349.525 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: DHFR, huDHFR / Plasmid: pET41a(+) / Production host: Homo sapiens (human) / Gene: DHFR, huDHFR / Plasmid: pET41a(+) / Production host:  #2: Chemical | #3: Chemical | ChemComp-1QZ / | #4: Chemical | ChemComp-FOL / | #5: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.31 Å3/Da / Density % sol: 46.81 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 0.1M Tris-HCl, 0.2-0.3M lithium sulfate, 25-32%(w/v) PEG 4000, pH 7.5, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X4A / Wavelength: 0.97016 Å / Beamline: X4A / Wavelength: 0.97016 Å |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Jun 12, 2012 |
| Radiation | Monochromator: ADSC Quantum 4R / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97016 Å / Relative weight: 1 |
| Reflection | Resolution: 1.45→50 Å / Num. all: 92682 / Num. obs: 92033 / % possible obs: 99.3 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 8 % |
| Reflection shell | Resolution: 1.45→1.49 Å / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2C2S Resolution: 1.45→16.096 Å / SU ML: 0.13 / σ(F): 1.35 / Phase error: 19.62 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.45→16.096 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller





















 PDBj
PDBj