[English] 日本語
 Yorodumi
Yorodumi- PDB-4kak: Crystal structure of human dihydrofolate reductase complexed with... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4kak | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human dihydrofolate reductase complexed with NADPH and 6-ethyl-5-[(3S)-3-[3-methoxy-5-(pyridine-4-yl)phenyl]but-1-yn-1-yl]pyrimidine-2,4-diamine (UCP1006) | ||||||
 Components Components | Dihydrofolate reductase | ||||||
 Keywords Keywords | Oxidoreductase/oxidoreductase inhibitor / Oxidoreductase / 5 / 6 / 7 / 8-tetrahydrofolate / NADP+ / 8-dihydrofolate / Hydride shift / Oxidoreductase-oxidoreductase inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of removal of superoxide radicals / tetrahydrobiopterin biosynthetic process / Metabolism of folate and pterines / tetrahydrofolate metabolic process / response to methotrexate / sequence-specific mRNA binding / folic acid binding / axon regeneration / dihydrofolate metabolic process / G1/S-Specific Transcription ...regulation of removal of superoxide radicals / tetrahydrobiopterin biosynthetic process / Metabolism of folate and pterines / tetrahydrofolate metabolic process / response to methotrexate / sequence-specific mRNA binding / folic acid binding / axon regeneration / dihydrofolate metabolic process / G1/S-Specific Transcription / dihydrofolate reductase / dihydrofolate reductase activity / folic acid metabolic process / tetrahydrofolate biosynthetic process / NADPH binding / Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation / one-carbon metabolic process / mRNA regulatory element binding translation repressor activity / NADP binding / negative regulation of translation / mRNA binding / mitochondrion / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Lamb, K.M. / Anderson, A.C. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2013 Journal: Biochemistry / Year: 2013Title: Elucidating features that drive the design of selective antifolates using crystal structures of human dihydrofolate reductase. Authors: Lamb, K.M. / G-Dayanandan, N. / Wright, D.L. / Anderson, A.C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4kak.cif.gz 4kak.cif.gz | 105.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4kak.ent.gz pdb4kak.ent.gz | 80.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4kak.json.gz 4kak.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ka/4kak https://data.pdbj.org/pub/pdb/validation_reports/ka/4kak ftp://data.pdbj.org/pub/pdb/validation_reports/ka/4kak ftp://data.pdbj.org/pub/pdb/validation_reports/ka/4kak | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4kbnC  4kd7C  4kebC  4kfjC  2c2sS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| 2 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 21349.525 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Strain: RP11-399L5 / Gene: DHFR, huDHFR / Plasmid: pET41a(+) / Production host: Homo sapiens (human) / Strain: RP11-399L5 / Gene: DHFR, huDHFR / Plasmid: pET41a(+) / Production host:  |
|---|
-Non-polymers , 5 types, 360 molecules 

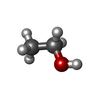






| #2: Chemical | | #3: Chemical | #4: Chemical | ChemComp-EOH / #5: Chemical | ChemComp-CA / #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.32 Å3/Da / Density % sol: 46.99 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 0.1M Tris-HCl pH 7.4, 0.2M Lithium sulfate, 30% (w/v) PEG 4000, 8% (v/v) ethanol, 11mM CaCl2, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE / Date: Jun 11, 2011 / Details: Varimax optics |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→46.82 Å / Num. all: 37102 / Num. obs: 37016 / % possible obs: 99.8 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 3.95 % / Biso Wilson estimate: 31.21 Å2 / Rmerge(I) obs: 0.061 / Net I/σ(I): 8.3 |
| Reflection shell | Resolution: 1.8→1.86 Å / Redundancy: 3.88 % / Rmerge(I) obs: 0.492 / Mean I/σ(I) obs: 1 / Rsym value: 0.554 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2C2S Resolution: 1.8→32.077 Å / Occupancy max: 1 / Occupancy min: 0.16 / FOM work R set: 0.7549 / SU ML: 0.27 / Phase error: 30.18 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 18.1734 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→32.077 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 13
|
 Movie
Movie Controller
Controller















 PDBj
PDBj














