+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4iwr | ||||||
|---|---|---|---|---|---|---|---|
| Title | C.Esp1396I bound to a 25 base pair operator site | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSCRIPTION/DNA / Restriction-modification / helix-turn-helix / transcriptional regulation / DNA / TRANSCRIPTION-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Enterobacter sp. (bacteria) Enterobacter sp. (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.4 Å molecular replacement / Resolution: 2.4 Å | ||||||
 Authors Authors | Martin, R.N.A. / McGeehan, J.E. / Ball, N.J. / Streeter, S.D. / Thresh, S.-J. / Kneale, G.G. | ||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.F / Year: 2013 Journal: Acta Crystallogr.,Sect.F / Year: 2013Title: Structural analysis of DNA-protein complexes regulating the restriction-modification system Esp1396I. Authors: Martin, R.N. / McGeehan, J.E. / Ball, N.J. / Streeter, S.D. / Thresh, S.J. / Kneale, G.G. #1:  Journal: Nucleic Acids Res. / Year: 2012 Journal: Nucleic Acids Res. / Year: 2012Title: Recognition of dual symmetry by the controller protein C.Esp1396I based on the structure of the transcriptional activation complex. Authors: McGeehan, J.E. / Ball, N.J. / Streeter, S.D. / Thresh, S.J. / Kneale, G.G. #2:  Journal: Nucleic Acids Res. / Year: 2012 Journal: Nucleic Acids Res. / Year: 2012Title: The structural basis of differential DNA sequence recognition by restriction-modification controller proteins. Authors: Ball, N.J. / McGeehan, J.E. / Streeter, S.D. / Thresh, S.J. / Kneale, G.G. #3:  Journal: Acta Crystallogr.,Sect.D / Year: 2009 Journal: Acta Crystallogr.,Sect.D / Year: 2009Title: Structure of the restriction-modification controller protein C.Esp1396I. Authors: Ball, N. / Streeter, S.D. / Kneale, G.G. / McGeehan, J.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4iwr.cif.gz 4iwr.cif.gz | 245.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4iwr.ent.gz pdb4iwr.ent.gz | 195.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4iwr.json.gz 4iwr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/iw/4iwr https://data.pdbj.org/pub/pdb/validation_reports/iw/4iwr ftp://data.pdbj.org/pub/pdb/validation_reports/iw/4iwr ftp://data.pdbj.org/pub/pdb/validation_reports/iw/4iwr | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4i8tC  3s8qS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 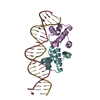
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 9521.175 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Enterobacter sp. (bacteria) / Strain: RFL1396 / Gene: esp1396IC / Plasmid: pET28 / Production host: Enterobacter sp. (bacteria) / Strain: RFL1396 / Gene: esp1396IC / Plasmid: pET28 / Production host:  #2: DNA chain | Mass: 7718.992 Da / Num. of mol.: 2 / Source method: obtained synthetically / Details: 25 base pair operator (OL) #3: DNA chain | Mass: 7634.976 Da / Num. of mol.: 2 / Source method: obtained synthetically / Details: 25 base pair operator (OL) (complimentary strand) #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.11 Å3/Da / Density % sol: 41.78 % |
|---|---|
| Crystal grow | Temperature: 289 K / Method: vapor diffusion, hanging drop / pH: 4 Details: 0.1 M PCB buffer, 20 % v/v PEG 1500, 10 mM spermidine , pH 4, VAPOR DIFFUSION, HANGING DROP, temperature 289K |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-4 / Wavelength: 0.933 Å / Beamline: ID14-4 / Wavelength: 0.933 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Nov 6, 2008 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: Si(111) double crystal monochromator / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.933 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.4→38.863 Å / Num. obs: 21045 / % possible obs: 95.6 % / Observed criterion σ(I): -3 / Biso Wilson estimate: 59.528 Å2 / Rmerge(I) obs: 0.048 / Net I/σ(I): 13.49 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR | Model details: Phaser MODE: MR_AUTO
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: pdb entry 3S8Q Resolution: 2.4→38.86 Å / Cor.coef. Fo:Fc: 0.954 / Cor.coef. Fo:Fc free: 0.914 / WRfactor Rfree: 0.2534 / WRfactor Rwork: 0.1912 / Occupancy max: 1 / Occupancy min: 0.5 / FOM work R set: 0.7826 / SU B: 26.222 / SU ML: 0.274 / SU R Cruickshank DPI: 0.7697 / SU Rfree: 0.3069 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.77 / ESU R Free: 0.307 / Stereochemistry target values: MAXIMUM LIKELIHOOD
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 128.46 Å2 / Biso mean: 67.385 Å2 / Biso min: 6.39 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.4→38.86 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.4→2.462 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller


















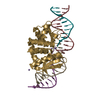
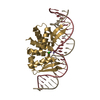


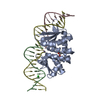

 PDBj
PDBj








































