[English] 日本語
 Yorodumi
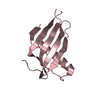
Yorodumi- PDB-3x0f: Crystal structure of the ectodomain of mouse CD81 large extracell... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3x0f | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the ectodomain of mouse CD81 large extracellular loop (mCD81-LEL) | ||||||
 Components Components | CD81 antigen | ||||||
 Keywords Keywords | CELL ADHESION / helical bundle / disulfide bond / immune cell adhesion / morphology / activation / proliferation / differentiation / Membrane | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of adaptive immune memory response / positive regulation of protein catabolic process in the vacuole / macrophage fusion / CD4-positive, alpha-beta T cell costimulation / positive regulation of B cell receptor signaling pathway / osteoclast fusion / myoblast fusion involved in skeletal muscle regeneration / positive regulation of T cell activation via T cell receptor contact with antigen bound to MHC molecule on antigen presenting cell / positive regulation of inflammatory response to antigenic stimulus / regulation of macrophage migration ...positive regulation of adaptive immune memory response / positive regulation of protein catabolic process in the vacuole / macrophage fusion / CD4-positive, alpha-beta T cell costimulation / positive regulation of B cell receptor signaling pathway / osteoclast fusion / myoblast fusion involved in skeletal muscle regeneration / positive regulation of T cell activation via T cell receptor contact with antigen bound to MHC molecule on antigen presenting cell / positive regulation of inflammatory response to antigenic stimulus / regulation of macrophage migration / Regulation of Complement cascade / positive regulation of B cell activation / immunological synapse formation / tetraspanin-enriched microdomain / transferrin receptor binding / positive regulation of T-helper 2 cell cytokine production / positive regulation of protein exit from endoplasmic reticulum / protein localization to lysosome / humoral immune response mediated by circulating immunoglobulin / MHC class II protein binding / regulation of cell motility / positive regulation of CD4-positive, alpha-beta T cell proliferation / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / cholesterol binding / positive regulation of T cell receptor signaling pathway / immunological synapse / cellular response to low-density lipoprotein particle stimulus / positive regulation of receptor clustering / positive regulation of B cell proliferation / protein localization to plasma membrane / regulation of protein stability / receptor internalization / integrin binding / virus receptor activity / basolateral plasma membrane / positive regulation of MAPK cascade / positive regulation of transcription by RNA polymerase II / extracellular exosome / membrane / plasma membrane / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.471 Å MOLECULAR REPLACEMENT / Resolution: 1.471 Å | ||||||
 Authors Authors | Zhang, M. / Cui, S. | ||||||
 Citation Citation |  Journal: Faseb J. / Year: 2015 Journal: Faseb J. / Year: 2015Title: An intramolecular bond at cluster of differentiation 81 ectodomain is important for hepatitis C virus entry. Authors: Yang, W. / Zhang, M. / Chi, X. / Liu, X. / Qin, B. / Cui, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3x0f.cif.gz 3x0f.cif.gz | 132.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3x0f.ent.gz pdb3x0f.ent.gz | 103.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3x0f.json.gz 3x0f.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/x0/3x0f https://data.pdbj.org/pub/pdb/validation_reports/x0/3x0f ftp://data.pdbj.org/pub/pdb/validation_reports/x0/3x0f ftp://data.pdbj.org/pub/pdb/validation_reports/x0/3x0f | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3x0eC  3x0gC  1g8qS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 10280.641 Da / Num. of mol.: 2 / Fragment: large extracellular loop, UNP residues 113-202 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Chemical | ChemComp-IPA / #3: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.95 Å3/Da / Density % sol: 36.94 % |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 5.5 Details: 0.1M Sodium Citrate pH 5.5, 5% PEG 1000, 35% isopropanol, VAPOR DIFFUSION, HANGING DROP, temperature 295K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06DA / Wavelength: 1.00002 Å / Beamline: X06DA / Wavelength: 1.00002 Å |
| Detector | Type: PSI PILATUS 6M / Detector: PIXEL / Date: Feb 10, 2014 / Details: mirrors |
| Radiation | Monochromator: Bartels Monochromator Crystal Type Si (111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.00002 Å / Relative weight: 1 |
| Reflection | Resolution: 1.471→44.617 Å / Num. all: 52822 / Num. obs: 52310 / % possible obs: 99 % / Observed criterion σ(F): -3 / Observed criterion σ(I): -3 / Redundancy: 3.3 % / Biso Wilson estimate: 31.568 Å2 / Rmerge(I) obs: 0.023 / Net I/σ(I): 22.63 |
| Reflection shell | Resolution: 1.47→1.56 Å / Redundancy: 3.18 % / Rmerge(I) obs: 0.469 / Mean I/σ(I) obs: 2.09 / Num. unique all: 8532 / % possible all: 97.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1G8Q Resolution: 1.471→36.231 Å / SU ML: 0.24 / Cross valid method: THROUGHOUT / σ(F): 1.19 / Phase error: 23.64 / Stereochemistry target values: ML Details: THE STRUCTURE FACTOR FILE CONTAINS FRIEDEL PAIRS IN F_PLUS/MINUS COLUMNS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.98 Å / VDW probe radii: 1.2 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 63.02 Å2 / ksol: 0.402 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 43.626 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.471→36.231 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 10
|
 Movie
Movie Controller
Controller












 PDBj
PDBj








