+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7ins | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | STRUCTURE OF PORCINE INSULIN COCRYSTALLIZED WITH CLUPEINE Z | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | HORMONE | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of lipoprotein lipase activity / Insulin processing / IRS activation / Signal attenuation / Insulin receptor signalling cascade / Signaling by Insulin receptor / Synthesis, secretion, and deacylation of Ghrelin / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / Insulin receptor recycling / response to L-arginine ...positive regulation of lipoprotein lipase activity / Insulin processing / IRS activation / Signal attenuation / Insulin receptor signalling cascade / Signaling by Insulin receptor / Synthesis, secretion, and deacylation of Ghrelin / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / Insulin receptor recycling / response to L-arginine / glycoprotein biosynthetic process / lactate biosynthetic process / positive regulation of glucose metabolic process / positive regulation of fatty acid biosynthetic process / lipoprotein biosynthetic process / COPI-mediated anterograde transport / negative regulation of glycogen catabolic process / positive regulation of nitric oxide mediated signal transduction / negative regulation of fatty acid metabolic process / negative regulation of feeding behavior / lipid biosynthetic process / positive regulation of respiratory burst / negative regulation of acute inflammatory response / alpha-beta T cell activation / positive regulation of dendritic spine maintenance / negative regulation of protein secretion / negative regulation of gluconeogenesis / positive regulation of glycogen biosynthetic process / fatty acid homeostasis / positive regulation of insulin receptor signaling pathway / negative regulation of respiratory burst involved in inflammatory response / negative regulation of lipid catabolic process / regulation of protein localization to plasma membrane / nitric oxide-cGMP-mediated signaling / negative regulation of reactive oxygen species biosynthetic process / insulin-like growth factor receptor binding / neuron projection maintenance / positive regulation of mitotic nuclear division / positive regulation of glycolytic process / positive regulation of DNA replication / positive regulation of cytokine production / acute-phase response / positive regulation of D-glucose import across plasma membrane / positive regulation of protein secretion / insulin receptor binding / wound healing / hormone activity / negative regulation of protein catabolic process / positive regulation of protein localization to nucleus / vasodilation / glucose metabolic process / insulin receptor signaling pathway / glucose homeostasis / protease binding / positive regulation of canonical NF-kappaB signal transduction / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of MAPK cascade / positive regulation of cell migration / G protein-coupled receptor signaling pathway / negative regulation of gene expression / positive regulation of cell population proliferation / extracellular space / identical protein binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2 Å X-RAY DIFFRACTION / Resolution: 2 Å | |||||||||
 Authors Authors | Balschmidt, P. / Hansen, F.B. / Dodson, E. / Dodson, G. / Korber, F. | |||||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.B / Year: 1991 Journal: Acta Crystallogr.,Sect.B / Year: 1991Title: Structure of porcine insulin cocrystallized with clupeine Z. Authors: Balschmidt, P. / Hansen, F.B. / Dodson, E.J. / Dodson, G.G. / Korber, F. #1:  Journal: Nature / Year: 1989 Journal: Nature / Year: 1989Title: Phenol Stabilizes More Helix in a New Symmetrical Zinc Insulin Hexamer Authors: Derewenda, U. / Derewenda, Z. / Dodson, E.J. / Dodson, G.G. / Reynolds, C.D. / Smith, G.D. / Sparks, C. / Swenson, D. #2:  Journal: Philos.Trans.R.Soc.London,Ser.B / Year: 1988 Journal: Philos.Trans.R.Soc.London,Ser.B / Year: 1988Title: The Structure of 2Zn Pig Insulin Crystals at 1.5 Angstroms Resolution Authors: Baker, E.N. / Blundell, T.L. / Cutfield, J.F. / Cutfield, S.M. / Dodson, E.J. / Dodson, G.G. / Hodgkin, D.M.C. / Hubbard, R.E. / Isaacs, N.W. / Reynolds, C.D. / Sakabe, K. / Sakabe, N. / Vijayan, N.M. | |||||||||
| History |
| |||||||||
| Remark 650 | HELIX THE B CHAIN HELIX IS EXTENDED TO THE FIRST SIX RESIDUES OF THE B CHAIN, THEREBY RUNNING FROM ...HELIX THE B CHAIN HELIX IS EXTENDED TO THE FIRST SIX RESIDUES OF THE B CHAIN, THEREBY RUNNING FROM B 1 TO B 20. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7ins.cif.gz 7ins.cif.gz | 53.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7ins.ent.gz pdb7ins.ent.gz | 36.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7ins.json.gz 7ins.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/in/7ins https://data.pdbj.org/pub/pdb/validation_reports/in/7ins ftp://data.pdbj.org/pub/pdb/validation_reports/in/7ins ftp://data.pdbj.org/pub/pdb/validation_reports/in/7ins | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 |
| ||||||||
| 6 | 
| ||||||||
| 7 | 
| ||||||||
| 8 | 
| ||||||||
| 9 | 
| ||||||||
| 10 | 
| ||||||||
| 11 | 
| ||||||||
| 12 | 
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: GLY G 21 - GLY G 22 OMEGA ANGLE = 149.010 PEPTIDE BOND DEVIATES SIGNIFICANTLY FROM TRANS CONFORMATION 2: LYS G 33 - GLY G 35 OMEGA ANGLE = 303.917 PEPTIDE BOND DEVIATES SIGNIFICANTLY FROM TRANS CONFORMATION |
- Components
Components
-INSULIN (CHAIN ... , 2 types, 6 molecules ACEBDF
| #1: Protein/peptide | Mass: 2383.698 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  #2: Protein/peptide | Mass: 3403.927 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  |
|---|
-Protein/peptide , 1 types, 1 molecules G
| #3: Protein/peptide | Mass: 1379.692 Da / Num. of mol.: 1 / Source method: obtained synthetically |
|---|
-Non-polymers , 4 types, 115 molecules 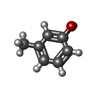




| #4: Chemical | | #5: Chemical | ChemComp-ZN / | #6: Chemical | Mass: 103.120 Da / Num. of mol.: 2 / Source method: obtained synthetically #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.27 Å3/Da / Density % sol: 45.72 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 277 K / pH: 7.3 / Method: batch method | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Reflection | *PLUS % possible obs: 75 % / Rmerge(I) obs: 0.071 |
|---|
- Processing
Processing
| Software | Name: PROLSQ / Classification: refinement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Rfactor obs: 0.194 / Highest resolution: 2 Å Details: CHAIN *G* IS THE GENERAL PROTAMINE CHAIN. THE PROTAMINE RESIDUES HAVE BEEN SELECTED TO FIT THE ELECTRON DENSITY WITHOUT ANY REFERENCE TO THE SEQUENCE OR CONTINUITY OF THE CLUPEINE Z. FOR ...Details: CHAIN *G* IS THE GENERAL PROTAMINE CHAIN. THE PROTAMINE RESIDUES HAVE BEEN SELECTED TO FIT THE ELECTRON DENSITY WITHOUT ANY REFERENCE TO THE SEQUENCE OR CONTINUITY OF THE CLUPEINE Z. FOR THIS REASON SOME ATOMS HAVE ZERO OCCUPANCY. THE SEQRES RECORDS FOR CHAIN *G* PRESENT THE ACTUAL SEQUENCE OF CLUPEINE Z. THE ATOM RECORDS FOR CHAIN *G* HAVE BEEN PRESENTED WITH RESIDUE NAME *UNK*. THE FOLLOWING PROTAMINE RESIDUES BELONG (LOOSELY) TOGETHER: ASSOCIATED WITH THE DIMER-DIMER INTERFACE G41, G 25, G 5, G 6, G 43; ASSOCIATED WITH THE TOP OF THE CENTRAL CAVITY G 8, G 21 - G 23, G 33. G 35 CA, C, O HAVE BEEN FITTED INTO DENSITY FOR THE UNIDENTIFIED ZINC LIGAND, NOTE THE OCCUPANCY FOR THE OTHER ATOMS OF G 35 IS ZERO. THERE IS AN APPROXIMATE THREEFOLD AXIS THROUGH (26.72, 26.72, 0) WITH DIRECTION COSINES (0.543, -0.543, 0.640). | ||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 2 Å
| ||||||||||||
| Software | *PLUS Name: PROLSQ / Classification: refinement | ||||||||||||
| Refinement | *PLUS Highest resolution: 2 Å / Lowest resolution: 10 Å / Num. reflection obs: 11522 / Rfactor obs: 0.194 | ||||||||||||
| Solvent computation | *PLUS | ||||||||||||
| Displacement parameters | *PLUS Biso mean: 33.7 Å2 |
 Movie
Movie Controller
Controller










 PDBj
PDBj








