+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3wnx | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
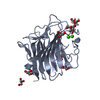
| Title | Crystal structure of ERGIC-53/MCFD2, Calcium/Man3-bound form | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | PROTEIN TRANSPORT / Beta-sandwich / EF-hand / Cargo receptor / Calcium binding / ER / ERGIC | |||||||||
| Function / homology |  Function and homology information Function and homology informationTransport to the Golgi and subsequent modification / positive regulation of organelle organization / : / Cargo concentration in the ER / COPII-coated ER to Golgi transport vesicle / endoplasmic reticulum organization / RHOD GTPase cycle / COPII-mediated vesicle transport / RHOC GTPase cycle / endoplasmic reticulum-Golgi intermediate compartment ...Transport to the Golgi and subsequent modification / positive regulation of organelle organization / : / Cargo concentration in the ER / COPII-coated ER to Golgi transport vesicle / endoplasmic reticulum organization / RHOD GTPase cycle / COPII-mediated vesicle transport / RHOC GTPase cycle / endoplasmic reticulum-Golgi intermediate compartment / Golgi organization / D-mannose binding / RHOG GTPase cycle / RHOA GTPase cycle / RAC2 GTPase cycle / RAC3 GTPase cycle / endoplasmic reticulum to Golgi vesicle-mediated transport / vesicle-mediated transport / endoplasmic reticulum-Golgi intermediate compartment membrane / sarcomere / ER to Golgi transport vesicle membrane / blood coagulation / unfolded protein binding / protein folding / protein transport / : / Golgi membrane / calcium ion binding / endoplasmic reticulum membrane / endoplasmic reticulum / Golgi apparatus / extracellular exosome / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.75 Å MOLECULAR REPLACEMENT / Resolution: 2.75 Å | |||||||||
 Authors Authors | Satoh, T. / Suzuki, K. / Yamaguchi, T. / Kato, K. | |||||||||
 Citation Citation |  Journal: Plos One / Year: 2014 Journal: Plos One / Year: 2014Title: Structural Basis for Disparate Sugar-Binding Specificities in the Homologous Cargo Receptors ERGIC-53 and VIP36 Authors: Satoh, T. / Suzuki, K. / Yamaguchi, T. / Kato, K. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3wnx.cif.gz 3wnx.cif.gz | 74.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3wnx.ent.gz pdb3wnx.ent.gz | 53 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3wnx.json.gz 3wnx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wn/3wnx https://data.pdbj.org/pub/pdb/validation_reports/wn/3wnx ftp://data.pdbj.org/pub/pdb/validation_reports/wn/3wnx ftp://data.pdbj.org/pub/pdb/validation_reports/wn/3wnx | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3whtC  3whuSC C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 27154.230 Da / Num. of mol.: 1 Fragment: Carbohydrate recognition domain (UNP residues 31-269) Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ERGIC53 / Plasmid: pCold-III / Production host: Homo sapiens (human) / Gene: ERGIC53 / Plasmid: pCold-III / Production host:  | ||||
|---|---|---|---|---|---|
| #2: Protein | Mass: 12056.186 Da / Num. of mol.: 1 / Fragment: UNP residues 67-146 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: MCFD2 / Plasmid: pET-16b / Production host: Homo sapiens (human) / Gene: MCFD2 / Plasmid: pET-16b / Production host:  | ||||
| #3: Polysaccharide | alpha-D-mannopyranose-(1-2)-alpha-D-mannopyranose / 2alpha-alpha-mannobiose | ||||
| #4: Chemical | ChemComp-CA / #5: Water | ChemComp-HOH / | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.95 Å3/Da / Density % sol: 36.82 % |
|---|---|
| Crystal grow | Temperature: 289 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 20% PEG5000 monomethyl ether, 100mM Bis-Tris, 10mM CaCl2, 10mM alpha2-mannotriose, pH 6.5, VAPOR DIFFUSION, HANGING DROP, temperature 289K |
-Data collection
| Diffraction | Mean temperature: 95 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Photon Factory Photon Factory  / Beamline: BL-5A / Wavelength: 1 Å / Beamline: BL-5A / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Nov 23, 2013 |
| Radiation | Monochromator: Si 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.75→50 Å / Num. all: 8058 / Num. obs: 8017 / % possible obs: 99.5 % / Observed criterion σ(I): -3 / Redundancy: 3.5 % / Biso Wilson estimate: 57.8 Å2 / Rmerge(I) obs: 0.079 / Net I/σ(I): 31.1 |
| Reflection shell | Resolution: 2.75→2.8 Å / Redundancy: 3.6 % / Rmerge(I) obs: 0.438 / Mean I/σ(I) obs: 4 / Num. unique all: 419 / % possible all: 99.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3WHU Resolution: 2.75→20 Å / Cor.coef. Fo:Fc: 0.956 / Cor.coef. Fo:Fc free: 0.92 / Occupancy max: 1 / Occupancy min: 1 / SU B: 23.63 / SU ML: 0.442 / Cross valid method: THROUGHOUT / ESU R Free: 0.439 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES: REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 165.87 Å2 / Biso mean: 79.0963 Å2 / Biso min: 36.59 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.75→20 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.75→2.82 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller



















 PDBj
PDBj









