[English] 日本語
 Yorodumi
Yorodumi- PDB-3ult: Crystal structure of an ice-binding protein from the perennial ry... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3ult | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of an ice-binding protein from the perennial ryegrass, Lolium perenne | ||||||
 Components Components | Ice recrystallization inhibition protein-like protein | ||||||
 Keywords Keywords | ANTIFREEZE PROTEIN / beta-solenoid / beta-roll / ice-binding / antifreeze / ice / extracellular | ||||||
| Function / homology |  Function and homology information Function and homology information: / Grass antifreeze beta roll / : / Serralysin-like metalloprotease, C-terminal / 2 Solenoid / Alkaline Protease, subunit P, domain 1 / Leucine-rich repeat-containing N-terminal, plant-type / Leucine rich repeat N-terminal domain / Serralysin-like metalloprotease, C-terminal / Leucine Rich Repeat ...: / Grass antifreeze beta roll / : / Serralysin-like metalloprotease, C-terminal / 2 Solenoid / Alkaline Protease, subunit P, domain 1 / Leucine-rich repeat-containing N-terminal, plant-type / Leucine rich repeat N-terminal domain / Serralysin-like metalloprotease, C-terminal / Leucine Rich Repeat / Leucine-rich repeat / Leucine-rich repeat domain superfamily / Mainly Beta Similarity search - Domain/homology | ||||||
| Biological species |  Lolium perenne (perennial ryegrass) Lolium perenne (perennial ryegrass) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SIRAS / Resolution: 1.4 Å SIRAS / Resolution: 1.4 Å | ||||||
 Authors Authors | Middleton, A.J. / Faucher, F. / Campbell, R.L. / Davies, P.L. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2012 Journal: J.Mol.Biol. / Year: 2012Title: Antifreeze protein from freeze-tolerant grass has a beta-roll fold with an irregularly structured ice-binding site. Authors: Middleton, A.J. / Marshall, C.B. / Faucher, F. / Bar-Dolev, M. / Braslavsky, I. / Campbell, R.L. / Walker, V.K. / Davies, P.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3ult.cif.gz 3ult.cif.gz | 100.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3ult.ent.gz pdb3ult.ent.gz | 80.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3ult.json.gz 3ult.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  3ult_validation.pdf.gz 3ult_validation.pdf.gz | 461.2 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  3ult_full_validation.pdf.gz 3ult_full_validation.pdf.gz | 463.7 KB | Display | |
| Data in XML |  3ult_validation.xml.gz 3ult_validation.xml.gz | 14 KB | Display | |
| Data in CIF |  3ult_validation.cif.gz 3ult_validation.cif.gz | 20.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ul/3ult https://data.pdbj.org/pub/pdb/validation_reports/ul/3ult ftp://data.pdbj.org/pub/pdb/validation_reports/ul/3ult ftp://data.pdbj.org/pub/pdb/validation_reports/ul/3ult | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 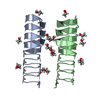
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 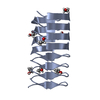
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 13503.053 Da / Num. of mol.: 2 / Fragment: UNP residues 137-254 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Lolium perenne (perennial ryegrass) / Gene: IRI3 / Plasmid: pET24a+ / Production host: Lolium perenne (perennial ryegrass) / Gene: IRI3 / Plasmid: pET24a+ / Production host:  #2: Chemical | #3: Chemical | ChemComp-EOH / #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 2 X-RAY DIFFRACTION / Number of used crystals: 2 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.19 Å3/Da / Density % sol: 43.91 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 4.2 Details: 45-50% Ethanol, 100 mM Phosphate-citrate, 0-5% PEG 1000, pH 4.2, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X6A / Wavelength: 1.13, 1.55 / Beamline: X6A / Wavelength: 1.13, 1.55 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 270 / Detector: CCD / Date: Feb 14, 2009 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Number: 51177 / Rmerge(I) obs: 0.073 / D res high: 2.1 Å / Num. obs: 23468 / % possible obs: 87.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diffraction reflection shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.4→20 Å / Num. obs: 45098 / % possible obs: 97.9 % / Observed criterion σ(F): -3 / Observed criterion σ(I): -3 / Redundancy: 5.7 % / Biso Wilson estimate: 17.982 Å2 / Rmerge(I) obs: 0.076 / Rsym value: 0.057 / Net I/σ(I): 16.83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
-Phasing
| Phasing | Method:  SIRAS SIRAS |
|---|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SIRAS / Resolution: 1.4→19.423 Å / Occupancy max: 1 / Occupancy min: 0.01 / SU ML: 0.28 / σ(F): 2 / Phase error: 20 / Stereochemistry target values: ML SIRAS / Resolution: 1.4→19.423 Å / Occupancy max: 1 / Occupancy min: 0.01 / SU ML: 0.28 / σ(F): 2 / Phase error: 20 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.41 Å / VDW probe radii: 0.6 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 64.897 Å2 / ksol: 0.493 e/Å3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 86.65 Å2 / Biso mean: 17.308 Å2 / Biso min: 6.82 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.4→19.423 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 16
|
 Movie
Movie Controller
Controller








 PDBj
PDBj