| Entry | Database: PDB / ID: 3twx
|
|---|
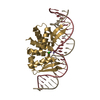
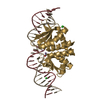
| Title | Crystal structure of ARC4 from human Tankyrase 2 in complex with peptide from human FNBP1 (chimeric peptide) |
|---|
 Components Components | |
|---|
 Keywords Keywords | SIGNALING PROTEIN/PEPTIDE / ankyrin repeat / protein-protein interaction / substrate recruitment / poly(ADP-ribosyl)ation / SIGNALING PROTEIN-PEPTIDE complex |
|---|
| Function / homology |  Function and homology information Function and homology information
XAV939 stabilizes AXIN / positive regulation of telomere capping / NAD+ ADP-ribosyltransferase / protein auto-ADP-ribosylation / protein localization to chromosome, telomeric region / negative regulation of telomere maintenance via telomere lengthening / NAD+-protein-aspartate ADP-ribosyltransferase activity / protein poly-ADP-ribosylation / NAD+-protein-glutamate ADP-ribosyltransferase activity / NAD+-protein mono-ADP-ribosyltransferase activity ...XAV939 stabilizes AXIN / positive regulation of telomere capping / NAD+ ADP-ribosyltransferase / protein auto-ADP-ribosylation / protein localization to chromosome, telomeric region / negative regulation of telomere maintenance via telomere lengthening / NAD+-protein-aspartate ADP-ribosyltransferase activity / protein poly-ADP-ribosylation / NAD+-protein-glutamate ADP-ribosyltransferase activity / NAD+-protein mono-ADP-ribosyltransferase activity / pericentriolar material / Transferases; Glycosyltransferases; Pentosyltransferases / NAD+ poly-ADP-ribosyltransferase activity / positive regulation of telomere maintenance via telomerase / nucleotidyltransferase activity / TCF dependent signaling in response to WNT / Degradation of AXIN / Wnt signaling pathway / Regulation of PTEN stability and activity / protein polyubiquitination / positive regulation of canonical Wnt signaling pathway / nuclear envelope / chromosome, telomeric region / Ub-specific processing proteases / Golgi membrane / perinuclear region of cytoplasm / enzyme binding / metal ion binding / nucleus / cytoplasm / cytosolSimilarity search - Function Ankyrin repeat-containing domain / Ankyrin repeats (many copies) / Poly(ADP-ribose) polymerase catalytic domain / Poly(ADP-ribose) polymerase, catalytic domain / PARP catalytic domain profile. / SAM domain (Sterile alpha motif) / SAM domain profile. / Sterile alpha motif. / Sterile alpha motif domain / Sterile alpha motif/pointed domain superfamily ...Ankyrin repeat-containing domain / Ankyrin repeats (many copies) / Poly(ADP-ribose) polymerase catalytic domain / Poly(ADP-ribose) polymerase, catalytic domain / PARP catalytic domain profile. / SAM domain (Sterile alpha motif) / SAM domain profile. / Sterile alpha motif. / Sterile alpha motif domain / Sterile alpha motif/pointed domain superfamily / Ankyrin repeat / Ankyrin repeats (3 copies) / Ankyrin repeat profile. / Ankyrin repeat region circular profile. / ankyrin repeats / Ankyrin repeat / Ankyrin repeat-containing domain superfamily / Serine Threonine Protein Phosphatase 5, Tetratricopeptide repeat / Alpha Horseshoe / Mainly AlphaSimilarity search - Domain/homology |
|---|
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å |
|---|
 Authors Authors | Guettler, S. / Sicheri, F. |
|---|
 Citation Citation |  Journal: Cell(Cambridge,Mass.) / Year: 2011 Journal: Cell(Cambridge,Mass.) / Year: 2011
Title: Structural basis and sequence rules for substrate recognition by tankyrase explain the basis for cherubism disease.
Authors: Guettler, S. / Larose, J. / Petsalaki, E. / Gish, G. / Scotter, A. / Pawson, T. / Rottapel, R. / Sicheri, F. |
|---|
| History | | Deposition | Sep 22, 2011 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Dec 7, 2011 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Dec 28, 2011 | Group: Database references |
|---|
| Revision 1.2 | Mar 26, 2025 | Group: Data collection / Database references ...Data collection / Database references / Derived calculations / Structure summary
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature / struct_conn / struct_ref_seq_dif / struct_site
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_conn.pdbx_leaving_atom_flag / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å
MOLECULAR REPLACEMENT / Resolution: 1.8 Å  Authors
Authors Citation
Citation Journal: Cell(Cambridge,Mass.) / Year: 2011
Journal: Cell(Cambridge,Mass.) / Year: 2011 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 3twx.cif.gz
3twx.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb3twx.ent.gz
pdb3twx.ent.gz PDB format
PDB format 3twx.json.gz
3twx.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/tw/3twx
https://data.pdbj.org/pub/pdb/validation_reports/tw/3twx ftp://data.pdbj.org/pub/pdb/validation_reports/tw/3twx
ftp://data.pdbj.org/pub/pdb/validation_reports/tw/3twx






 Links
Links Assembly
Assembly



 Components
Components Homo sapiens (human) / Gene: PARP5B, TANK2, TNKL, TNKS2 / Plasmid: pETM-30 / Production host:
Homo sapiens (human) / Gene: PARP5B, TANK2, TNKL, TNKS2 / Plasmid: pETM-30 / Production host: 
 Homo sapiens (human)
Homo sapiens (human)






 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 24-ID-E / Wavelength: 0.9792 Å
/ Beamline: 24-ID-E / Wavelength: 0.9792 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 1.8→35.638 Å / SU ML: 0.47 / σ(F): 0.29 / Phase error: 21.59 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 1.8→35.638 Å / SU ML: 0.47 / σ(F): 0.29 / Phase error: 21.59 / Stereochemistry target values: ML Movie
Movie Controller
Controller










 PDBj
PDBj





