[English] 日本語
 Yorodumi
Yorodumi- PDB-3pfs: PWWP Domain of Human Bromodomain and PHD finger-containing protein 3 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3pfs | ||||||
|---|---|---|---|---|---|---|---|
| Title | PWWP Domain of Human Bromodomain and PHD finger-containing protein 3 | ||||||
 Components Components | Bromodomain and PHD finger-containing protein 3 | ||||||
 Keywords Keywords | PROTEIN BINDING / Structural Genomics / Structural Genomics Consortium / SGC / PWWP Domain | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of developmental process / MOZ/MORF histone acetyltransferase complex / regulation of hemopoiesis / histone acetyltransferase complex / Regulation of TP53 Activity through Acetylation / positive regulation of DNA replication / Platelet degranulation / HATs acetylate histones / chromatin remodeling / regulation of DNA-templated transcription ...regulation of developmental process / MOZ/MORF histone acetyltransferase complex / regulation of hemopoiesis / histone acetyltransferase complex / Regulation of TP53 Activity through Acetylation / positive regulation of DNA replication / Platelet degranulation / HATs acetylate histones / chromatin remodeling / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / extracellular region / zinc ion binding / nucleus / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.9 Å molecular replacement / Resolution: 1.9 Å | ||||||
 Authors Authors | Lam, R. / Zeng, H. / Kania, J. / Bountra, C. / Weigelt, J. / Arrowsmith, C.H. / Edwards, A.M. / Min, J. / Wu, H. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: Plos One / Year: 2011 Journal: Plos One / Year: 2011Title: Structural and histone binding ability characterizations of human PWWP domains. Authors: Wu, H. / Zeng, H. / Lam, R. / Tempel, W. / Amaya, M.F. / Xu, C. / Dombrovski, L. / Qiu, W. / Wang, Y. / Min, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3pfs.cif.gz 3pfs.cif.gz | 119.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3pfs.ent.gz pdb3pfs.ent.gz | 92 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3pfs.json.gz 3pfs.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  3pfs_validation.pdf.gz 3pfs_validation.pdf.gz | 445.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  3pfs_full_validation.pdf.gz 3pfs_full_validation.pdf.gz | 445.7 KB | Display | |
| Data in XML |  3pfs_validation.xml.gz 3pfs_validation.xml.gz | 12.7 KB | Display | |
| Data in CIF |  3pfs_validation.cif.gz 3pfs_validation.cif.gz | 17.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pf/3pfs https://data.pdbj.org/pub/pdb/validation_reports/pf/3pfs ftp://data.pdbj.org/pub/pdb/validation_reports/pf/3pfs ftp://data.pdbj.org/pub/pdb/validation_reports/pf/3pfs | HTTPS FTP |
-Related structure data
| Related structure data |  3eaeC  3l42C  3llrC  3lyiC  3mo8C  3pmiC  3qbyC  3qj6C  3qkjC  2x4wS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 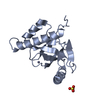
| |||||||||
| 2 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 18187.018 Da / Num. of mol.: 2 / Fragment: PWWP domain (UNP Residues 1056-1195) Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: BRPF3, KIAA1286 / Plasmid: pET28-MHL / Production host: Homo sapiens (human) / Gene: BRPF3, KIAA1286 / Plasmid: pET28-MHL / Production host:  #2: Chemical | #3: Chemical | ChemComp-SO4 / | #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.91 Å3/Da / Density % sol: 35.66 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 30% PEG4000, 0.2M Ammonium Sulfate, 0.1M Sodium Cacodylate pH 6.5, VAPOR DIFFUSION, SITTING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.97924 Å / Beamline: 19-ID / Wavelength: 0.97924 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Aug 18, 2010 Details: Rosenbaum-Rock high-resolution double-crystal monochromator | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: double-crystal monochromator / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.97924 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.9→33.631 Å / Num. obs: 22879 / % possible obs: 99.9 % / Redundancy: 6 % / Rmerge(I) obs: 0.091 / Χ2: 1.005 / Net I/σ(I): 9.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR |
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2X4W Resolution: 1.9→33.631 Å / Cor.coef. Fo:Fc: 0.938 / Cor.coef. Fo:Fc free: 0.909 / WRfactor Rfree: 0.261 / WRfactor Rwork: 0.213 / Occupancy max: 1 / Occupancy min: 0.5 / SU B: 6.492 / SU ML: 0.104 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.171 / ESU R Free: 0.161 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT U VALUES : WITH TLS ADDED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL WITH MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 64.71 Å2 / Biso mean: 22.3843 Å2 / Biso min: 8.69 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→33.631 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj










