[English] 日本語
 Yorodumi
Yorodumi- PDB-3ovl: Structure of an amyloid forming peptide VQIVYK from the TAU prote... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3ovl | ||||||
|---|---|---|---|---|---|---|---|
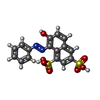
| Title | Structure of an amyloid forming peptide VQIVYK from the TAU protein in complex with orange G | ||||||
 Components Components | Microtubule-associated protein | ||||||
 Keywords Keywords | PROTEIN FIBRIL / amyloid-like protofibril in complex with a small-molecule binder | ||||||
| Function / homology |  Function and homology information Function and homology informationplus-end-directed organelle transport along microtubule / histone-dependent DNA binding / negative regulation of protein localization to mitochondrion / neurofibrillary tangle / microtubule lateral binding / axonal transport / tubulin complex / positive regulation of protein localization to synapse / negative regulation of tubulin deacetylation / phosphatidylinositol bisphosphate binding ...plus-end-directed organelle transport along microtubule / histone-dependent DNA binding / negative regulation of protein localization to mitochondrion / neurofibrillary tangle / microtubule lateral binding / axonal transport / tubulin complex / positive regulation of protein localization to synapse / negative regulation of tubulin deacetylation / phosphatidylinositol bisphosphate binding / generation of neurons / rRNA metabolic process / axonal transport of mitochondrion / regulation of mitochondrial fission / axon development / regulation of chromosome organization / central nervous system neuron development / intracellular distribution of mitochondria / minor groove of adenine-thymine-rich DNA binding / lipoprotein particle binding / microtubule polymerization / negative regulation of mitochondrial membrane potential / regulation of microtubule polymerization / dynactin binding / main axon / apolipoprotein binding / protein polymerization / axolemma / glial cell projection / Caspase-mediated cleavage of cytoskeletal proteins / regulation of microtubule polymerization or depolymerization / negative regulation of mitochondrial fission / neurofibrillary tangle assembly / positive regulation of axon extension / regulation of cellular response to heat / synapse assembly / Activation of AMPK downstream of NMDARs / regulation of long-term synaptic depression / positive regulation of superoxide anion generation / positive regulation of protein localization / cellular response to brain-derived neurotrophic factor stimulus / supramolecular fiber organization / cytoplasmic microtubule organization / somatodendritic compartment / regulation of calcium-mediated signaling / axon cytoplasm / positive regulation of microtubule polymerization / astrocyte activation / phosphatidylinositol binding / nuclear periphery / stress granule assembly / protein phosphatase 2A binding / regulation of microtubule cytoskeleton organization / cellular response to reactive oxygen species / microglial cell activation / Hsp90 protein binding / cellular response to nerve growth factor stimulus / synapse organization / protein homooligomerization / PKR-mediated signaling / regulation of synaptic plasticity / response to lead ion / SH3 domain binding / microtubule cytoskeleton organization / memory / cytoplasmic ribonucleoprotein granule / neuron projection development / cell-cell signaling / single-stranded DNA binding / protein-folding chaperone binding / cellular response to heat / microtubule cytoskeleton / actin binding / growth cone / cell body / double-stranded DNA binding / protein-macromolecule adaptor activity / sequence-specific DNA binding / microtubule binding / dendritic spine / amyloid fibril formation / microtubule / learning or memory / neuron projection / regulation of autophagy / membrane raft / axon / negative regulation of gene expression / neuronal cell body / DNA damage response / dendrite / protein kinase binding / enzyme binding / mitochondrion / DNA binding / RNA binding / extracellular region / identical protein binding / nucleus / plasma membrane Similarity search - Function | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.81 Å molecular replacement / Resolution: 1.81 Å | ||||||
 Authors Authors | Landau, M. / Eisenberg, D. | ||||||
 Citation Citation |  Journal: Plos Biol. / Year: 2011 Journal: Plos Biol. / Year: 2011Title: Towards a pharmacophore for amyloid. Authors: Landau, M. / Sawaya, M.R. / Faull, K.F. / Laganowsky, A. / Jiang, L. / Sievers, S.A. / Liu, J. / Barrio, J.R. / Eisenberg, D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3ovl.cif.gz 3ovl.cif.gz | 12.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3ovl.ent.gz pdb3ovl.ent.gz | 7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3ovl.json.gz 3ovl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ov/3ovl https://data.pdbj.org/pub/pdb/validation_reports/ov/3ovl ftp://data.pdbj.org/pub/pdb/validation_reports/ov/3ovl ftp://data.pdbj.org/pub/pdb/validation_reports/ov/3ovl | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 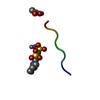
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | x 6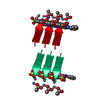
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
| ||||||||
| Details | The biological unit is a pair of beta sheets with orange G interrelating between two pairs of sheets. One sheet is constructed from chain A and unit cell translations along the b direction (i.e. X,Y+1,Z; X,Y+2,Z; X,Y+3,Z, etc.). The second sheet is constructed from -X-1/2,1/2+Y,-Z;-X-1/2,3/2+Y,-Z;-X-1/2,5/2+Y,-Z; etc. The other pairs of sheets will be contracted from -X,Y,-Z+1, -X,Y+1,-Z+1,-X,Y+2,-Z+1; etc. and from X+1/2,1/2+Y,Z+1, X+1/2,3/2+Y,Z+1, X+1/2,5/2+Y,Z+1; etc. |
- Components
Components
| #1: Protein/peptide | Mass: 749.917 Da / Num. of mol.: 1 / Fragment: VQIVYK (residues 306-311) / Source method: obtained synthetically / Details: VQIVYK (residues 306-311) from Tau, synthesized / References: UniProt: P10636 | ||||||
|---|---|---|---|---|---|---|---|
| #2: Chemical | | #3: Chemical | ChemComp-ACY / | #4: Chemical | ChemComp-ORA / | #5: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.91 Å3/Da / Density % sol: 35.66 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, hanging drop Details: reservoir contained 0.1M Zinc Acetate dihydrate, 18% PEG 3350, vapor diffusion, hanging drop, temperature 291K |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-E / Wavelength: 0.9792 Å / Beamline: 24-ID-E / Wavelength: 0.9792 Å | ||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Nov 17, 2008 | ||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9792 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.8→90 Å / Num. all: 587 / Num. obs: 587 / % possible obs: 87.6 % / Observed criterion σ(I): -3 / Redundancy: 2.4 % / Biso Wilson estimate: 19.9 Å2 / Rmerge(I) obs: 0.179 / Χ2: 1.071 / Net I/σ(I): 4.5 | ||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR | Model details: Phaser MODE: MR_AUTO
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.81→26.82 Å / Cor.coef. Fo:Fc: 0.918 / Cor.coef. Fo:Fc free: 0.964 / WRfactor Rfree: 0.306 / WRfactor Rwork: 0.2514 / Occupancy max: 1 / Occupancy min: 0.25 / FOM work R set: 0.7697 / SU B: 5.919 / SU ML: 0.163 / SU R Cruickshank DPI: 0.3608 / SU Rfree: 0.2072 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.207 / Stereochemistry target values: MAXIMUM LIKELIHOOD MOLECULAR REPLACEMENT / Resolution: 1.81→26.82 Å / Cor.coef. Fo:Fc: 0.918 / Cor.coef. Fo:Fc free: 0.964 / WRfactor Rfree: 0.306 / WRfactor Rwork: 0.2514 / Occupancy max: 1 / Occupancy min: 0.25 / FOM work R set: 0.7697 / SU B: 5.919 / SU ML: 0.163 / SU R Cruickshank DPI: 0.3608 / SU Rfree: 0.2072 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.207 / Stereochemistry target values: MAXIMUM LIKELIHOODDetails: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 36.92 Å2 / Biso mean: 20.2828 Å2 / Biso min: 6.3 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.81→26.82 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.805→2.017 Å / Total num. of bins used: 5
|
 Movie
Movie Controller
Controller








 PDBj
PDBj