[English] 日本語
 Yorodumi
Yorodumi- PDB-2y3i: The structure of the fully closed conformation of human PGK in co... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2y3i | ||||||
|---|---|---|---|---|---|---|---|
| Title | The structure of the fully closed conformation of human PGK in complex with L-ADP, 3PG and the TSA aluminium tetrafluoride | ||||||
 Components Components | PHOSPHOGLYCERATE KINASE 1 | ||||||
 Keywords Keywords | TRANSFERASE / AIDS / HEPATITIS / CANCER / L-NUCLEOSIDE ANALOGUES / GLYCOLYSIS | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of pyruvate decarboxylation to acetyl-CoA / Manipulation of host energy metabolism / phosphoglycerate kinase / phosphoglycerate kinase activity / protein-disulfide reductase [NAD(P)H] activity / Gluconeogenesis / canonical glycolysis / Glycolysis / plasminogen activation / epithelial cell differentiation ...negative regulation of pyruvate decarboxylation to acetyl-CoA / Manipulation of host energy metabolism / phosphoglycerate kinase / phosphoglycerate kinase activity / protein-disulfide reductase [NAD(P)H] activity / Gluconeogenesis / canonical glycolysis / Glycolysis / plasminogen activation / epithelial cell differentiation / negative regulation of angiogenesis / glycolytic process / gluconeogenesis / ADP binding / cellular response to hypoxia / transmembrane transporter binding / non-specific serine/threonine protein kinase / membrane raft / mitochondrial matrix / protein serine kinase activity / protein serine/threonine kinase activity / extracellular space / extracellular exosome / ATP binding / metal ion binding / membrane / cytosol Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.9 Å MOLECULAR REPLACEMENT / Resolution: 2.9 Å | ||||||
 Authors Authors | Bowler, M.W. / Chaloin, L. / Lionne, C. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2011 Journal: J.Mol.Biol. / Year: 2011Title: Interaction of Human 3-Phosphoglycerate Kinase with its Two Substrates: Is Substrate Antagonism a Kinetic Advantage? Authors: Lallemand, P. / Chaloin, L. / Roy, B. / Barman, T. / Bowler, M.W. / Lionne, C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2y3i.cif.gz 2y3i.cif.gz | 245.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2y3i.ent.gz pdb2y3i.ent.gz | 196.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2y3i.json.gz 2y3i.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/y3/2y3i https://data.pdbj.org/pub/pdb/validation_reports/y3/2y3i ftp://data.pdbj.org/pub/pdb/validation_reports/y3/2y3i ftp://data.pdbj.org/pub/pdb/validation_reports/y3/2y3i | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2ybeC  2wzcS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 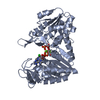
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Ens-ID: 1 / Refine code: 1
NCS oper: (Code: given Matrix: (-0.998, 0.057, -0.031), Vector: |
- Components
Components
-Protein , 1 types, 2 molecules AD
| #1: Protein | Mass: 44577.500 Da / Num. of mol.: 2 / Fragment: RESIDUES 1-416 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Production host: HOMO SAPIENS (human) / Production host:  |
|---|
-Non-polymers , 6 types, 26 molecules 

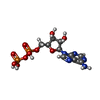







| #2: Chemical | | #3: Chemical | #4: Chemical | #5: Chemical | #6: Chemical | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Nonpolymer details | ADENOSINE-5'-DIPHOSPHAT |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.29 Å3/Da / Density % sol: 46.33 % Description: DATA WERE COLLECTED FROM 10UM NEEDLE USING A HELICAL DATA COLLECTION STRATEGY IN AN AREA DEFINED BY DIFFRACTION CARTOGRAPHY |
|---|---|
| Crystal grow | pH: 6.5 / Details: 26% PEG 2000MME, 0.1 M BIS/TRIS PH 6.5 . |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-2 / Wavelength: 0.873 / Beamline: ID23-2 / Wavelength: 0.873 |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Sep 3, 2010 / Details: KB |
| Radiation | Monochromator: SI111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.873 Å / Relative weight: 1 |
| Reflection | Resolution: 2.9→46.24 Å / Num. obs: 17028 / % possible obs: 90.7 % / Observed criterion σ(I): 3 / Redundancy: 3 % / Biso Wilson estimate: 60 Å2 / Rmerge(I) obs: 0.13 / Net I/σ(I): 7.7 |
| Reflection shell | Resolution: 2.9→3.06 Å / Redundancy: 3.1 % / Rmerge(I) obs: 0.51 / Mean I/σ(I) obs: 2.3 / % possible all: 99.1 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2WZC Resolution: 2.9→20 Å / Cor.coef. Fo:Fc: 0.87 / Cor.coef. Fo:Fc free: 0.855 / SU B: 38.264 / SU ML: 0.569 / Cross valid method: THROUGHOUT / ESU R Free: 0.58 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 31.534 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.9→20 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




















 PDBj
PDBj













