[English] 日本語
 Yorodumi
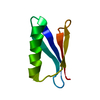
Yorodumi- PDB-2oed: GB3 solution structure obtained by refinement of X-ray structure ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2oed | ||||||
|---|---|---|---|---|---|---|---|
| Title | GB3 solution structure obtained by refinement of X-ray structure with dipolar couplings | ||||||
 Components Components | Immunoglobulin G-binding protein G | ||||||
 Keywords Keywords | IMMUNE SYSTEM / residual dipolar couplings | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Streptococcus sp. 'group G' (bacteria) Streptococcus sp. 'group G' (bacteria) | ||||||
| Method | SOLUTION NMR / simulated annealing | ||||||
 Authors Authors | Ulmer, T.S. / Ramirez, B.E. / Delaglio, F. / Bax, A. / Grishaev, A. | ||||||
 Citation Citation |  Journal: J.Am.Chem.Soc. / Year: 2003 Journal: J.Am.Chem.Soc. / Year: 2003Title: Evaluation of backbone proton positions and dynamics in a small protein by liquid crystal NMR spectroscopy Authors: Ulmer, T.S. / Ramirez, B.E. / Delaglio, F. / Bax, A. #1:  Journal: J.Mol.Biol. / Year: 1994 Journal: J.Mol.Biol. / Year: 1994Title: The third IGG-binding domain from Streptococcal protein G. An analysis by X-ray crystallography of the structure alone and in a complex with FAB Authors: Derrick, J.P. / Wiggley, D.B. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2oed.cif.gz 2oed.cif.gz | 27 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2oed.ent.gz pdb2oed.ent.gz | 17.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2oed.json.gz 2oed.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oe/2oed https://data.pdbj.org/pub/pdb/validation_reports/oe/2oed ftp://data.pdbj.org/pub/pdb/validation_reports/oe/2oed ftp://data.pdbj.org/pub/pdb/validation_reports/oe/2oed | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Antibody | Mass: 6214.848 Da / Num. of mol.: 1 / Fragment: THIRD IGG-BINDING DOMAIN Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Streptococcus sp. 'group G' (bacteria) / Gene: spg / Plasmid: PET-11A / Production host: Streptococcus sp. 'group G' (bacteria) / Gene: spg / Plasmid: PET-11A / Production host:  |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Contents: 25 MM NAH2PO4/NA2HPO4, 0.2 MG/ML NAN3, 1.5 MM GB3 PROTEIN AND ALIGNING MEDIA, 90% H2O, 10% D2O Solvent system: 90% H2O/10% D2O |
|---|---|
| Sample conditions | Ionic strength: 0.05 / pH: 6.5 / Pressure: ambient / Temperature: 300 K |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radiation wavelength | Relative weight: 1 | |||||||||||||||
| NMR spectrometer |
|
- Processing
Processing
| NMR software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: simulated annealing / Software ordinal: 1 Details: GB3 solution structure is obtained by refinement of the x-ray structure of gb3 (1igd) with c(alpha)-c', c'-n, c(alpha)-h(alpha) and N-H dipolar couplings measured in five aligning media ...Details: GB3 solution structure is obtained by refinement of the x-ray structure of gb3 (1igd) with c(alpha)-c', c'-n, c(alpha)-h(alpha) and N-H dipolar couplings measured in five aligning media (bicelle, peg, pf1 phage, negatively and positively charged polyacrylamide gels). Dipolar couplings originating on residues 10-11, 24-26, 39-41 and the n- and c-terminal residues were excluded, resulting in near-identity with the x-ray structure for these residues. This deposition corresponds to the refined-II structure of the primary citation that was refined against all 4 types of dipolar couplings in 5 media. The related depositions 1P7E and 1P7F correspond to the refined-III and refined-IV structures, respectively. The deposited structure was calculated with DYNAMO 2.1 following a short low-temperature molecular dynamics simulated annealing run starting from the regularized coordinates of the X-ray structure 1IGD using atomic coordinate restraints to the overlapping 3-residue segments of 1IGD. The force constants for the HN-N-C-Ca impropers were softened 10-fold relative to their standard settings. See the primary citation and the corresponding supporting information file for a detailed description of the refinement procedure. The deposited restraints file includes, in addition to the dipolar couplings and backbone H-bond distance restraints (XPLOR/CNS format), an Xplor-NIH script designed to mimic the deposited structure, and the force field parameter files. A set of overlapping 3-residue NCS restraint terms is used in this script in place of the Dynamo atomic coordinate restraints. | ||||||||||||
| NMR representative | Selection criteria: best agreement with dipolar restraints | ||||||||||||
| NMR ensemble | Conformer selection criteria: all calculated structures submitted Conformers calculated total number: 1 / Conformers submitted total number: 1 |
 Movie
Movie Controller
Controller











 PDBj
PDBj

 HSQC
HSQC