[English] 日本語
 Yorodumi
Yorodumi- PDB-2jcq: The hyaluronan binding domain of murine CD44 in a Type A complex ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2jcq | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The hyaluronan binding domain of murine CD44 in a Type A complex with an HA 8-mer | |||||||||
 Components Components | CD44 ANTIGEN | |||||||||
 Keywords Keywords | SUGAR BINDING PROTEIN / SUGAR-BINDING PROTEIN / HYAURONAN / LINK-DOMAIN / PROTEOGLYCAN / BLOOD GROUP ANTIGEN / LECTIN / ANTIGEN / MEMBRANE / RECEPTOR / SULFATION / GLYCOPROTEIN / C-TYPE LECTIN / CELL ADHESION / EXTRACELLULAR MATRIX / PYRROLIDONE CARBOXYLIC ACID / TRANSMEMBRANE / SUGAR- BINDING / PHOSPHORYLATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationHyaluronan degradation / hyaluronic acid binding / macrophage migration inhibitory factor receptor complex / negative regulation of regulatory T cell differentiation / Degradation of the extracellular matrix / Integrin cell surface interactions / regulation of lamellipodium morphogenesis / Cell surface interactions at the vascular wall / wound healing involved in inflammatory response / hyaluronan catabolic process ...Hyaluronan degradation / hyaluronic acid binding / macrophage migration inhibitory factor receptor complex / negative regulation of regulatory T cell differentiation / Degradation of the extracellular matrix / Integrin cell surface interactions / regulation of lamellipodium morphogenesis / Cell surface interactions at the vascular wall / wound healing involved in inflammatory response / hyaluronan catabolic process / branching involved in prostate gland morphogenesis / positive regulation of adaptive immune response / type II transforming growth factor beta receptor binding / negative regulation of mature B cell apoptotic process / channel regulator activity / negative regulation of CD4-positive, alpha-beta T cell proliferation / wound healing, spreading of cells / cargo receptor activity / branching involved in ureteric bud morphogenesis / negative regulation of intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / microvillus / negative regulation of DNA damage response, signal transduction by p53 class mediator / lamellipodium membrane / Neutrophil degranulation / receptor-mediated endocytosis / cell projection / negative regulation of inflammatory response / Wnt signaling pathway / cytokine-mediated signaling pathway / basolateral plasma membrane / positive regulation of ERK1 and ERK2 cascade / cell adhesion / apical plasma membrane / membrane raft / external side of plasma membrane / positive regulation of gene expression / cell surface / extracellular region / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.25 Å MOLECULAR REPLACEMENT / Resolution: 1.25 Å | |||||||||
 Authors Authors | Banerji, S. / Wright, A.J. / Noble, M.E.M. / Mahoney, D.J. / Campbell, I.D. / Day, A.J. / Jackson, D.G. | |||||||||
 Citation Citation |  Journal: Nat.Struct.Mol.Biol. / Year: 2008 Journal: Nat.Struct.Mol.Biol. / Year: 2008Title: Structures of the Cd44-Hyaluronan Complex Provide Insight Into a Fundamental Carbohydrate-Protein Interaction. Authors: Banerji, S. / Wright, A.J. / Noble, M.E.M. / Mahoney, D.J. / Campbell, I.D. / Day, A.J. / Jackson, D.G. | |||||||||
| History |
|
- Structure visualization
Structure visualization
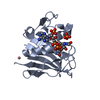
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2jcq.cif.gz 2jcq.cif.gz | 145.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2jcq.ent.gz pdb2jcq.ent.gz | 116 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2jcq.json.gz 2jcq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  2jcq_validation.pdf.gz 2jcq_validation.pdf.gz | 703.6 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  2jcq_full_validation.pdf.gz 2jcq_full_validation.pdf.gz | 706.2 KB | Display | |
| Data in XML |  2jcq_validation.xml.gz 2jcq_validation.xml.gz | 11 KB | Display | |
| Data in CIF |  2jcq_validation.cif.gz 2jcq_validation.cif.gz | 15.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jc/2jcq https://data.pdbj.org/pub/pdb/validation_reports/jc/2jcq ftp://data.pdbj.org/pub/pdb/validation_reports/jc/2jcq ftp://data.pdbj.org/pub/pdb/validation_reports/jc/2jcq | HTTPS FTP |
-Related structure data
| Related structure data |  2jcpC  2jcrC  1uuhS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 17129.047 Da / Num. of mol.: 1 / Fragment: HYALURONAN BINDING DOMAIN, RESIDUES 23-174 Source method: isolated from a genetically manipulated source Details: ENCODED RESIDUES 25-174, EQUIVALENT TO RESIDUES 1-151 OF THE MATURE PROTEIN, WITH ADDITIONAL RESIDUES M, N ADDED AT THE N-TERMINUS Source: (gene. exp.)   |
|---|---|
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-beta-D-glucopyranuronic acid-(1-3)-2-acetamido-2- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-beta-D-glucopyranuronic acid-(1-3)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-beta-D-glucopyranuronic acid-(1-3)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-beta-D-glucopyranuronic acid-(1-3)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
| #3: Chemical | ChemComp-GOL / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.12 Å3/Da / Density % sol: 42 % |
|---|---|
| Crystal grow | pH: 7 Details: CO-CRYSTALS OF THE CD44/HA8 COMPLEX WERE PREPARED AFTER ADDITION OF HA8 (2MM FINAL CONCENTRATION) TO THE 0.5MM PROTEIN SOLUTION FOLLOWED BY MIXING 1:1 WITH WELL SOLUTIONS CONTAINING 25% (W/V) ...Details: CO-CRYSTALS OF THE CD44/HA8 COMPLEX WERE PREPARED AFTER ADDITION OF HA8 (2MM FINAL CONCENTRATION) TO THE 0.5MM PROTEIN SOLUTION FOLLOWED BY MIXING 1:1 WITH WELL SOLUTIONS CONTAINING 25% (W/V) PEG 3350 AND 100MM NACL IN 100MM HEPES BUFFERED AT EITHER PH 7.0 OR PH 8.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-1 / Wavelength: 0.934 / Beamline: ID14-1 / Wavelength: 0.934 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Apr 1, 2004 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.934 Å / Relative weight: 1 |
| Reflection | Resolution: 1.25→23.42 Å / Num. obs: 36225 / % possible obs: 92.7 % / Observed criterion σ(I): 0 / Redundancy: 5.44 % / Rmerge(I) obs: 0.07 / Net I/σ(I): 6.75 |
| Reflection shell | Resolution: 1.25→1.32 Å / Redundancy: 2.65 % / Rmerge(I) obs: 0.42 / Mean I/σ(I) obs: 1.77 / % possible all: 56.2 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1UUH Resolution: 1.25→41.03 Å / Cor.coef. Fo:Fc: 0.976 / Cor.coef. Fo:Fc free: 0.97 / SU B: 1.234 / SU ML: 0.025 / Cross valid method: THROUGHOUT / ESU R: 0.043 / ESU R Free: 0.041 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 7.6 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.25→41.03 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj

















