Entry Database : PDB / ID : 2ibzTitle Yeast Cytochrome BC1 Complex with Stigmatellin (Ubiquinol-cytochrome c reductase complex ...) x 4 (Ubiquinol-cytochrome-c reductase complex core protein ...) x 2 Cytochrome b Cytochrome c1, heme protein, mitochondrial precursor Ubiquinol-cytochrome c reductase iron-sulfur subunit, mitochondrial precursor Variable Heavy chain of antibody fragment Variable Light chain of antibody fragment Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Mus musculus (house mouse)Saccharomyces cerevisiae (brewer's yeast)Method / / / Resolution : 2.3 Å Authors Hunte, C. Journal : J.Mol.Biol. / Year : 2007Title : A Comparison of Stigmatellin Conformations, Free and Bound to the Photosynthetic Reaction Center and the Cytochrome bc(1) Complex.Authors : Lancaster, C.R. / Hunte, C. / Kelley, J. / Trumpower, B.L. / Ditchfield, R. History Deposition Sep 12, 2006 Deposition site / Processing site Revision 1.0 Mar 20, 2007 Provider / Type Revision 1.1 May 1, 2008 Group Revision 1.2 Jul 13, 2011 Group Revision 1.3 Oct 18, 2017 Group / Refinement description / Category / software / Item Revision 2.0 Mar 3, 2021 Group Advisory / Atomic model ... Advisory / Atomic model / Data collection / Database references / Derived calculations / Non-polymer description / Structure summary Category atom_site / chem_comp ... atom_site / chem_comp / entity / pdbx_distant_solvent_atoms / pdbx_entity_nonpoly / pdbx_nonpoly_scheme / pdbx_struct_conn_angle / struct_conn / struct_conn_type / struct_ref_seq_dif / struct_site / struct_site_gen Item _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ... _atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.type_symbol / _chem_comp.formula / _chem_comp.formula_weight / _chem_comp.id / _chem_comp.name / _chem_comp.pdbx_synonyms / _entity.formula_weight / _entity.pdbx_description / _pdbx_entity_nonpoly.comp_id / _pdbx_entity_nonpoly.name / _pdbx_nonpoly_scheme.mon_id / _pdbx_nonpoly_scheme.pdb_mon_id / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr2_auth_comp_id / _pdbx_struct_conn_angle.ptnr2_auth_seq_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr2_label_atom_id / _pdbx_struct_conn_angle.ptnr2_label_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_conn_type.id / _struct_ref_seq_dif.details / _struct_site.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id / _struct_site_gen.auth_comp_id / _struct_site_gen.label_comp_id Revision 2.1 Nov 13, 2024 Group Advisory / Data collection ... Advisory / Data collection / Database references / Structure summary Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature / pdbx_unobs_or_zero_occ_residues Item / _database_2.pdbx_database_accession
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information

 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MIR / Resolution: 2.3 Å
MIR / Resolution: 2.3 Å  Authors
Authors Citation
Citation Journal: J.Mol.Biol. / Year: 2007
Journal: J.Mol.Biol. / Year: 2007 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2ibz.cif.gz
2ibz.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2ibz.ent.gz
pdb2ibz.ent.gz PDB format
PDB format 2ibz.json.gz
2ibz.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ib/2ibz
https://data.pdbj.org/pub/pdb/validation_reports/ib/2ibz ftp://data.pdbj.org/pub/pdb/validation_reports/ib/2ibz
ftp://data.pdbj.org/pub/pdb/validation_reports/ib/2ibz Links
Links Assembly
Assembly
 Components
Components














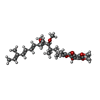






 X-RAY DIFFRACTION / Number of used crystals: 17
X-RAY DIFFRACTION / Number of used crystals: 17  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ESRF
ESRF  / Beamline: ID14-3
/ Beamline: ID14-3 Processing
Processing MIR / Resolution: 2.3→14.96 Å / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
MIR / Resolution: 2.3→14.96 Å / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber Movie
Movie Controller
Controller
















 PDBj
PDBj























