[English] 日本語
 Yorodumi
Yorodumi- PDB-2bag: 3D Structure of Torpedo californica acetylcholinesterase complexe... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2bag | ||||||
|---|---|---|---|---|---|---|---|
| Title | 3D Structure of Torpedo californica acetylcholinesterase complexed with Ganstigmine | ||||||
 Components Components | Acetylcholinesterase | ||||||
 Keywords Keywords | HYDROLASE / Serine hydrolase / Cholinesterase / Neurotransmitter cleavage / anti-Alzheimer drug | ||||||
| Function / homology |  Function and homology information Function and homology informationacetylcholine catabolic process in synaptic cleft / acetylcholinesterase / choline metabolic process / acetylcholinesterase activity / synaptic cleft / side of membrane / synapse / extracellular space / plasma membrane Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å MOLECULAR REPLACEMENT / Resolution: 2.4 Å | ||||||
 Authors Authors | Lamba, D. / Bartolucci, C. / Siotto, M. / Racchi, M. / Villetti, G. / Delcanale, M. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2006 Journal: J.Med.Chem. / Year: 2006Title: Structural Determinants of Torpedo californica Acetylcholinesterase Inhibition by the Novel and Orally Active Carbamate Based Anti-Alzheimer Drug Ganstigmine (CHF-2819) Authors: Bartolucci, C. / Siotto, M. / Ghidini, E. / Amari, G. / Bolzoni, P.T. / Racchi, M. / Villetti, G. / Delcanale, M. / Lamba, D. #1:  Journal: Biochemistry / Year: 1999 Journal: Biochemistry / Year: 1999Title: Back Door opening implied by the crystal structure of a carbamoylated acetylcholinesterase Authors: Bartolucci, C. / Perola, E. / Cellai, L. / Brufani, M. / Lamba, D. #2:  Journal: Biochemistry / Year: 2002 Journal: Biochemistry / Year: 2002Title: Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine Authors: Bar-On, P. / Millard, C.B. / Harel, M. / Dvir, H. / Enz, A. / Sussman, J.L. / Silman, I. #3: Journal: Biochim.Biophys.Acta / Year: 1997 Title: Long chain analogs of physostigmine as potential drugs for Alzheimer's disease: new insights into the mechanism of action in the inhibition of acetylcholinesterase Authors: Perola, E. / Cellai, L. / Lamba, D. / Filocamo, L. / Brufani, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2bag.cif.gz 2bag.cif.gz | 128.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2bag.ent.gz pdb2bag.ent.gz | 97.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2bag.json.gz 2bag.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ba/2bag https://data.pdbj.org/pub/pdb/validation_reports/ba/2bag ftp://data.pdbj.org/pub/pdb/validation_reports/ba/2bag ftp://data.pdbj.org/pub/pdb/validation_reports/ba/2bag | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2aceS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Sugars , 2 types, 3 molecules A

| #1: Protein | Mass: 61325.090 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  Organ: Eletric organ / Variant: G2 form / Tissue: Electroplaque / References: UniProt: P04058, acetylcholinesterase |
|---|---|
| #2: Sugar |
-Non-polymers , 4 types, 278 molecules 
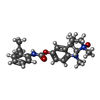





| #3: Chemical | ChemComp-1PE / | ||
|---|---|---|---|
| #4: Chemical | ChemComp-GSG / | ||
| #5: Chemical | | #6: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4 Å3/Da / Density % sol: 69.2 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 6 Details: 40% PEG200, 100mM MES, pH 6.0, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ELETTRA ELETTRA  / Beamline: 5.2R / Wavelength: 1 Å / Beamline: 5.2R / Wavelength: 1 Å |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: May 31, 1998 / Details: Three-segment Pt-coated toroidal mirror |
| Radiation | Monochromator: Double Crystal (Si111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.4→27.93 Å / Num. all: 37817 / Num. obs: 37817 / % possible obs: 96.4 % / Observed criterion σ(F): -3 / Observed criterion σ(I): 0 / Redundancy: 6 % / Biso Wilson estimate: 35.3 Å2 / Rmerge(I) obs: 0.063 / Rsym value: 0.057 / Net I/σ(I): 8.6 |
| Reflection shell | Resolution: 2.4→2.44 Å / Redundancy: 5.1 % / Rmerge(I) obs: 0.47 / Mean I/σ(I) obs: 1.9 / Num. unique all: 2303 / Rsym value: 0.422 / % possible all: 97.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2ACE Resolution: 2.4→27.93 Å / Rfactor Rfree error: 0.004 / Data cutoff high absF: 1599796.36 / Data cutoff low absF: 0 / Isotropic thermal model: Overall anisotropic / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: Flat model / Bsol: 50.11 Å2 / ksol: 0.365 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 45.6 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.4→27.93 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.4→2.55 Å / Rfactor Rfree error: 0.012 / Total num. of bins used: 6
|
 Movie
Movie Controller
Controller


























 PDBj
PDBj




