[English] 日本語
 Yorodumi
Yorodumi- PDB-1tco: TERNARY COMPLEX OF A CALCINEURIN A FRAGMENT, CALCINEURIN B, FKBP1... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1tco | ||||||
|---|---|---|---|---|---|---|---|
| Title | TERNARY COMPLEX OF A CALCINEURIN A FRAGMENT, CALCINEURIN B, FKBP12 AND THE IMMUNOSUPPRESSANT DRUG FK506 (TACROLIMUS) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | COMPLEX (HYDROLASE/ISOMERASE) / COMPLEX (HYDROLASE-ISOMERASE) / IMMUNOSUPPRESSANT / COMPLEX (HYDROLASE-ISOMERASE) complex | ||||||
| Function / homology |  Function and homology information Function and homology informationmTORC1-mediated signalling / TGF-beta receptor signaling activates SMADs / CLEC7A (Dectin-1) induces NFAT activation / regulation of activin receptor signaling pathway / Calcineurin activates NFAT / FCERI mediated Ca+2 mobilization / Activation of BAD and translocation to mitochondria / calcium-dependent protein serine/threonine phosphatase regulator activity / regulation of cell proliferation involved in kidney morphogenesis / positive regulation of glomerulus development ...mTORC1-mediated signalling / TGF-beta receptor signaling activates SMADs / CLEC7A (Dectin-1) induces NFAT activation / regulation of activin receptor signaling pathway / Calcineurin activates NFAT / FCERI mediated Ca+2 mobilization / Activation of BAD and translocation to mitochondria / calcium-dependent protein serine/threonine phosphatase regulator activity / regulation of cell proliferation involved in kidney morphogenesis / positive regulation of glomerulus development / negative regulation of signaling / positive regulation of saliva secretion / calmodulin-dependent protein phosphatase activity / calcineurin complex / macrolide binding / activin receptor binding / renal filtration / calcineurin-NFAT signaling cascade / regulation of skeletal muscle contraction by regulation of release of sequestered calcium ion / cytoplasmic side of membrane / transforming growth factor beta receptor binding / activin binding / TGFBR1 LBD Mutants in Cancer / type I transforming growth factor beta receptor binding / signaling receptor inhibitor activity / negative regulation of activin receptor signaling pathway / heart trabecula formation / positive regulation of osteoclast differentiation / I-SMAD binding / negative regulation of release of sequestered calcium ion into cytosol / dephosphorylation / regulation of amyloid precursor protein catabolic process / terminal cisterna / ryanodine receptor complex / response to caffeine / Ca2+ pathway / protein peptidyl-prolyl isomerization / 'de novo' protein folding / ventricular cardiac muscle tissue morphogenesis / protein dephosphorylation / protein-serine/threonine phosphatase / FK506 binding / negative regulation of ryanodine-sensitive calcium-release channel activity / SMAD binding / negative regulation of protein phosphorylation / TGF-beta receptor signaling activates SMADs / regulation of ryanodine-sensitive calcium-release channel activity / positive regulation of activated T cell proliferation / mTORC1-mediated signalling / positive regulation of protein binding / Calcineurin activates NFAT / epidermis development / regulation of immune response / positive regulation of osteoblast differentiation / T cell proliferation / phosphatase binding / heart morphogenesis / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / keratinocyte differentiation / calcium channel inhibitor activity / skeletal muscle fiber development / supramolecular fiber organization / release of sequestered calcium ion into cytosol / sarcoplasmic reticulum membrane / muscle contraction / T cell activation / positive regulation of protein ubiquitination / sarcoplasmic reticulum / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) / protein maturation / peptidylprolyl isomerase / calcium channel regulator activity / peptidyl-prolyl cis-trans isomerase activity / negative regulation of transforming growth factor beta receptor signaling pathway / response to calcium ion / sarcolemma / modulation of chemical synaptic transmission / Z disc / cytokine-mediated signaling pathway / SARS-CoV-1 activates/modulates innate immune responses / regulation of protein localization / protein folding / protein refolding / amyloid fibril formation / dendritic spine / Potential therapeutics for SARS / transmembrane transporter binding / calmodulin binding / positive regulation of canonical NF-kappaB signal transduction / calcium ion binding / protein homodimerization activity / extracellular exosome / metal ion binding / membrane / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.5 Å X-RAY DIFFRACTION / Resolution: 2.5 Å | ||||||
 Authors Authors | Griffith, J.P. / Kim, J.L. / Kim, E.E. / Sintchak, M.D. / Thomson, J.A. / Fitzgibbon, M.J. / Fleming, M.A. / Caron, P.R. / Hsiao, K. / Navia, M.A. | ||||||
 Citation Citation |  Journal: Cell(Cambridge,Mass.) / Year: 1995 Journal: Cell(Cambridge,Mass.) / Year: 1995Title: X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Authors: Griffith, J.P. / Kim, J.L. / Kim, E.E. / Sintchak, M.D. / Thomson, J.A. / Fitzgibbon, M.J. / Fleming, M.A. / Caron, P.R. / Hsiao, K. / Navia, M.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1tco.cif.gz 1tco.cif.gz | 163.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1tco.ent.gz pdb1tco.ent.gz | 123.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1tco.json.gz 1tco.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tc/1tco https://data.pdbj.org/pub/pdb/validation_reports/tc/1tco ftp://data.pdbj.org/pub/pdb/validation_reports/tc/1tco ftp://data.pdbj.org/pub/pdb/validation_reports/tc/1tco | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-SERINE/THREONINE PHOSPHATASE ... , 2 types, 2 molecules AB
| #1: Protein | Mass: 43012.969 Da / Num. of mol.: 1 Fragment: CHAIN A IS THE CATALYTIC SUBUNIT, RESIDUES 18 -392. CHAIN B IS THE REGULATORY SUBUNIT, RESIDUES 1 - 169 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P48452, protein-serine/threonine phosphatase |
|---|---|
| #2: Protein | Mass: 19191.709 Da / Num. of mol.: 1 Fragment: CHAIN A IS THE CATALYTIC SUBUNIT, RESIDUES 18 -392. CHAIN B IS THE REGULATORY SUBUNIT, RESIDUES 1 - 169 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P63099, protein-serine/threonine phosphatase |
-Protein , 1 types, 1 molecules C
| #3: Protein | Mass: 11750.392 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P62942, UniProt: P18203*PLUS, peptidylprolyl isomerase |
|---|
-Non-polymers , 7 types, 719 molecules 




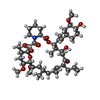







| #4: Chemical | ChemComp-ZN / | ||||||
|---|---|---|---|---|---|---|---|
| #5: Chemical | ChemComp-FE / | ||||||
| #6: Chemical | ChemComp-PO4 / | ||||||
| #7: Chemical | ChemComp-CA / #8: Chemical | ChemComp-MYR / | #9: Chemical | ChemComp-FK5 / | #10: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.35 Å3/Da / Density % sol: 63.34 % | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 4 ℃ / Method: vapor diffusion, hanging drop | ||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction source | Wavelength: 1.5418 |
|---|---|
| Detector | Type: RIGAKU RAXIS / Detector: IMAGE PLATE / Date: 1995 |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Num. obs: 41097 / % possible obs: 91.3 % / Observed criterion σ(I): 0 / Redundancy: 3 % |
| Reflection | *PLUS Highest resolution: 2.3 Å / Rmerge(I) obs: 0.05 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.5→7 Å / σ(F): 1.5
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→7 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


















