+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1lxi | ||||||
|---|---|---|---|---|---|---|---|
| Title | Refinement of BMP7 crystal structure | ||||||
 Components Components | BONE MORPHOGENETIC PROTEIN 7 | ||||||
 Keywords Keywords | HORMONE/GROWTH FACTOR / Cystine-knot growth factor / HORMONE-GROWTH FACTOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of prostatic bud formation / negative regulation of mesenchymal cell apoptotic process involved in nephron morphogenesis / negative regulation of glomerular mesangial cell proliferation / mesenchymal cell apoptotic process involved in nephron morphogenesis / positive regulation of cardiac neural crest cell migration involved in outflow tract morphogenesis / positive regulation of hyaluranon cable assembly / chorio-allantoic fusion / metanephric mesenchyme morphogenesis / nephrogenic mesenchyme morphogenesis / embryonic skeletal joint morphogenesis ...negative regulation of prostatic bud formation / negative regulation of mesenchymal cell apoptotic process involved in nephron morphogenesis / negative regulation of glomerular mesangial cell proliferation / mesenchymal cell apoptotic process involved in nephron morphogenesis / positive regulation of cardiac neural crest cell migration involved in outflow tract morphogenesis / positive regulation of hyaluranon cable assembly / chorio-allantoic fusion / metanephric mesenchyme morphogenesis / nephrogenic mesenchyme morphogenesis / embryonic skeletal joint morphogenesis / neural fold elevation formation / negative regulation of striated muscle cell apoptotic process / metanephric mesenchymal cell proliferation involved in metanephros development / mesenchyme development / positive regulation of epithelial cell differentiation / ameloblast differentiation / embryonic camera-type eye morphogenesis / monocyte aggregation / allantois development / mesonephros development / regulation of removal of superoxide radicals / pericardium morphogenesis / hindbrain development / mesenchymal cell differentiation / endocardial cushion formation / BMP receptor binding / pharyngeal system development / cellular response to BMP stimulus / heart trabecula morphogenesis / branching involved in salivary gland morphogenesis / embryonic pattern specification / metanephros development / negative regulation of mitotic nuclear division / positive regulation of heterotypic cell-cell adhesion / cartilage development / embryonic limb morphogenesis / negative regulation of non-canonical NF-kappaB signal transduction / response to vitamin D / positive regulation of dendrite development / cardiac muscle tissue development / ureteric bud development / Molecules associated with elastic fibres / regulation of branching involved in prostate gland morphogenesis / cardiac septum morphogenesis / negative regulation of neuron differentiation / branching morphogenesis of an epithelial tube / odontogenesis of dentin-containing tooth / dendrite development / negative regulation of cell cycle / mesoderm formation / positive regulation of SMAD protein signal transduction / epithelial to mesenchymal transition / negative regulation of Notch signaling pathway / positive regulation of bone mineralization / positive regulation of osteoblast differentiation / BMP signaling pathway / positive regulation of epithelial to mesenchymal transition / Transcriptional regulation of brown and beige adipocyte differentiation by EBF2 / neuron projection morphogenesis / positive regulation of brown fat cell differentiation / positive regulation of neuron differentiation / axon guidance / cytokine activity / protein serine/threonine kinase activator activity / skeletal system development / growth factor activity / response to peptide hormone / negative regulation of neurogenesis / osteoblast differentiation / response to estradiol / heparin binding / cellular response to hypoxia / vesicle / positive regulation of apoptotic process / negative regulation of DNA-templated transcription / positive regulation of gene expression / positive regulation of DNA-templated transcription / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular region Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | ||||||
 Authors Authors | Greenwald, J. / Groppe, J. / Kwiatkowski, W. / Choe, S. | ||||||
 Citation Citation |  Journal: Mol.Cell / Year: 2003 Journal: Mol.Cell / Year: 2003Title: The BMP7/ActRII Extracellular Domain Complex Provides New Insights into the Cooperative Nature of Receptor Assembly Authors: Greenwald, J. / Groppe, J. / Gray, P. / Wiater, E. / Kwiatkowski, W. / Vale, W. / Choe, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1lxi.cif.gz 1lxi.cif.gz | 37.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1lxi.ent.gz pdb1lxi.ent.gz | 25.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1lxi.json.gz 1lxi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lx/1lxi https://data.pdbj.org/pub/pdb/validation_reports/lx/1lxi ftp://data.pdbj.org/pub/pdb/validation_reports/lx/1lxi ftp://data.pdbj.org/pub/pdb/validation_reports/lx/1lxi | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1lx5C  1bmpS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 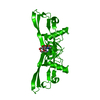
| ||||||||
| Unit cell |
| ||||||||
| Details | The second part of the biological assembly is generated by the two fold axis: x-y+1,-y+2,1/3-z |
- Components
Components
| #1: Protein | Mass: 15699.730 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Description: DHFR- / Gene: BMP7 / Cell (production host): OVARY CELLS / Production host: Homo sapiens (human) / Description: DHFR- / Gene: BMP7 / Cell (production host): OVARY CELLS / Production host:  |
|---|---|
| #2: Sugar | ChemComp-NAG / |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.94 Å3/Da / Density % sol: 68.8 % | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 4.5 Details: 20% MPD, 0.1M Sodium Acetate, pH 4.5, VAPOR DIFFUSION, HANGING DROP, temperature 277K | ||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 23 ℃ | ||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL9-1 / Wavelength: 0.98 Å / Beamline: BL9-1 / Wavelength: 0.98 Å |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Sep 1, 2000 / Details: mirror |
| Radiation | Monochromator: Si 311 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.98 Å / Relative weight: 1 |
| Reflection | Resolution: 2→25 Å / Num. all: 15770 / Num. obs: 15770 / % possible obs: 99.6 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 / Rsym value: 0.054 / Net I/σ(I): 16.9 |
| Reflection shell | Resolution: 2→2.08 Å / Rsym value: 0.333 / % possible all: 99.6 |
| Reflection | *PLUS Highest resolution: 2 Å / Lowest resolution: 100 Å / % possible obs: 93.3 % / Num. measured all: 67831 / Rmerge(I) obs: 0.054 |
| Reflection shell | *PLUS Highest resolution: 2 Å / % possible obs: 99.6 % / Rmerge(I) obs: 0.333 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 1BMP Resolution: 2→23.96 Å / Cor.coef. Fo:Fc: 0.934 / Cor.coef. Fo:Fc free: 0.934 / SU B: 4.128 / SU ML: 0.119 / Isotropic thermal model: Isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.137 / ESU R Free: 0.127 / Stereochemistry target values: Engh & Huber / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 35.983 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→23.96 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2→2.052 Å / Total num. of bins used: 20 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Version: 5 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2 Å / Lowest resolution: 40 Å / % reflection Rfree: 5 % / Rfactor Rfree: 0.244 / Rfactor Rwork: 0.228 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj





