[English] 日本語
 Yorodumi
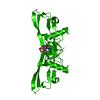
Yorodumi- PDB-1lx5: Crystal Structure of the BMP7/ActRII Extracellular Domain Complex -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1lx5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of the BMP7/ActRII Extracellular Domain Complex | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | GROWTH FACTOR/GROWTH FACTOR RECEPTOR / LIGAND-RECEPTOR COMPLEX / GROWTH FACTOR-GROWTH FACTOR RECEPTOR COMPLEX | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of prostatic bud formation / negative regulation of mesenchymal cell apoptotic process involved in nephron morphogenesis / negative regulation of glomerular mesangial cell proliferation / mesenchymal cell apoptotic process involved in nephron morphogenesis / positive regulation of cardiac neural crest cell migration involved in outflow tract morphogenesis / TGFBR3 regulates activin signaling / positive regulation of hyaluranon cable assembly / Signaling by Activin / inhibin binding / chorio-allantoic fusion ...negative regulation of prostatic bud formation / negative regulation of mesenchymal cell apoptotic process involved in nephron morphogenesis / negative regulation of glomerular mesangial cell proliferation / mesenchymal cell apoptotic process involved in nephron morphogenesis / positive regulation of cardiac neural crest cell migration involved in outflow tract morphogenesis / TGFBR3 regulates activin signaling / positive regulation of hyaluranon cable assembly / Signaling by Activin / inhibin binding / chorio-allantoic fusion / inhibin-betaglycan-ActRII complex / metanephric mesenchyme morphogenesis / nephrogenic mesenchyme morphogenesis / Signaling by BMP / activin receptor activity / penile erection / embryonic skeletal joint morphogenesis / activin receptor activity, type II / neural fold elevation formation / negative regulation of striated muscle cell apoptotic process / positive regulation of activin receptor signaling pathway / sperm ejaculation / metanephric mesenchymal cell proliferation involved in metanephros development / mesenchyme development / positive regulation of epithelial cell differentiation / ameloblast differentiation / embryonic camera-type eye morphogenesis / Sertoli cell proliferation / monocyte aggregation / allantois development / mesonephros development / BMP receptor activity / embryonic skeletal system development / regulation of removal of superoxide radicals / pericardium morphogenesis / hindbrain development / mesenchymal cell differentiation / activin receptor activity, type I / endocardial cushion formation / BMP receptor binding / pharyngeal system development / receptor protein serine/threonine kinase / activin binding / cellular response to BMP stimulus / heart trabecula morphogenesis / branching involved in salivary gland morphogenesis / embryonic pattern specification / metanephros development / negative regulation of mitotic nuclear division / gastrulation with mouth forming second / positive regulation of heterotypic cell-cell adhesion / cartilage development / regulation of nitric oxide biosynthetic process / embryonic limb morphogenesis / determination of left/right symmetry / negative regulation of non-canonical NF-kappaB signal transduction / response to vitamin D / positive regulation of dendrite development / anterior/posterior pattern specification / cardiac muscle tissue development / ureteric bud development / Molecules associated with elastic fibres / regulation of branching involved in prostate gland morphogenesis / growth factor binding / cardiac septum morphogenesis / negative regulation of neuron differentiation / branching morphogenesis of an epithelial tube / odontogenesis of dentin-containing tooth / mesoderm development / dendrite development / negative regulation of cell cycle / mesoderm formation / positive regulation of SMAD protein signal transduction / epithelial to mesenchymal transition / negative regulation of Notch signaling pathway / regulation of signal transduction / positive regulation of bone mineralization / positive regulation of osteoblast differentiation / BMP signaling pathway / positive regulation of epithelial to mesenchymal transition / Transcriptional regulation of brown and beige adipocyte differentiation by EBF2 / coreceptor activity / neuron projection morphogenesis / positive regulation of brown fat cell differentiation / positive regulation of neuron differentiation / axon guidance / positive regulation of erythrocyte differentiation / cytokine activity / protein serine/threonine kinase activator activity / skeletal system development / PDZ domain binding / growth factor activity / response to peptide hormone / negative regulation of neurogenesis / male gonad development / osteoblast differentiation / response to estradiol / heparin binding / spermatogenesis / cellular response to hypoxia Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MIR / Resolution: 3.3 Å MIR / Resolution: 3.3 Å | |||||||||
 Authors Authors | Greenwald, J. / Groppe, J. / Kwiatkowski, W. / Choe, S. | |||||||||
 Citation Citation |  Journal: Mol.Cell / Year: 2003 Journal: Mol.Cell / Year: 2003Title: The BMP7/ActRII Extracellular Domain Complex Provides New Insights into the Cooperative Nature of Receptor Assembly Authors: Greenwald, J. / Groppe, J. / Gray, P. / Wiater, E. / Kwiatkowski, W. / Vale, W. / Choe, S. | |||||||||
| History |
| |||||||||
| Remark 999 | SEQUENCE THE SEQEUNCE FOR ACVR2 IS TRUNCATED. THE PROTEIN IN THE CRYSTAL ENDS AT GLU 102. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1lx5.cif.gz 1lx5.cif.gz | 60.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1lx5.ent.gz pdb1lx5.ent.gz | 43.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1lx5.json.gz 1lx5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lx/1lx5 https://data.pdbj.org/pub/pdb/validation_reports/lx/1lx5 ftp://data.pdbj.org/pub/pdb/validation_reports/lx/1lx5 ftp://data.pdbj.org/pub/pdb/validation_reports/lx/1lx5 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Details | The second part of the biological assembly is generated by the two fold axis: y-x+1, y, -z+1 |
- Components
Components
| #1: Protein | Mass: 15699.730 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Description: DHFR- / Gene: BMP7 / Cell (production host): OVARY CELLS / Production host: Homo sapiens (human) / Description: DHFR- / Gene: BMP7 / Cell (production host): OVARY CELLS / Production host:  | ||
|---|---|---|---|
| #2: Protein | Mass: 12019.440 Da / Num. of mol.: 1 Fragment: Extracellular Ligand Binding Domain, C-terminal truncation Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pichia pastoris (fungus) / Strain (production host): SMD1168 / References: UniProt: P27038 Pichia pastoris (fungus) / Strain (production host): SMD1168 / References: UniProt: P27038 | ||
| #3: Polysaccharide | alpha-D-mannopyranose-(1-3)-[beta-D-mannopyranose-(1-4)][alpha-D-mannopyranose-(1-6)]beta-D- ...alpha-D-mannopyranose-(1-3)-[beta-D-mannopyranose-(1-4)][alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source | ||
| #4: Sugar | | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.69 Å3/Da / Density % sol: 73.79 % | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 296 K / Method: vapor diffusion, hanging drop / pH: 7 Details: 1M sodium acetate, 0.1M imidazole, pH 7, VAPOR DIFFUSION, HANGING DROP, temperature 296K | ||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 23 ℃ | ||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL11-1 / Wavelength: 1.03 Å / Beamline: BL11-1 / Wavelength: 1.03 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Feb 10, 2002 |
| Radiation | Monochromator: Si 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.03 Å / Relative weight: 1 |
| Reflection | Resolution: 3.3→30 Å / Num. all: 8320 / Num. obs: 8320 / % possible obs: 96.7 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 / Biso Wilson estimate: 145 Å2 / Rsym value: 0.055 / Net I/σ(I): 16.5 |
| Reflection shell | Resolution: 3.3→3.42 Å / Rsym value: 0.332 / % possible all: 98.6 |
| Reflection | *PLUS Lowest resolution: 100 Å / Num. measured all: 31531 / Rmerge(I) obs: 0.055 |
| Reflection shell | *PLUS % possible obs: 98.6 % / Rmerge(I) obs: 0.332 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MIR / Resolution: 3.3→27.12 Å / Cor.coef. Fo:Fc: 0.899 / Cor.coef. Fo:Fc free: 0.858 / SU B: 8.619 / SU ML: 0.151 / Isotropic thermal model: isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.949 / ESU R Free: 0.435 / Stereochemistry target values: Engh & Huber MIR / Resolution: 3.3→27.12 Å / Cor.coef. Fo:Fc: 0.899 / Cor.coef. Fo:Fc free: 0.858 / SU B: 8.619 / SU ML: 0.151 / Isotropic thermal model: isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.949 / ESU R Free: 0.435 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 69.6 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.3→27.12 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 3.3→3.386 Å / Total num. of bins used: 20 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: REFMAC / Version: 5 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 3.3 Å / Lowest resolution: 100 Å / % reflection Rfree: 5 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller












 PDBj
PDBj





