[English] 日本語
 Yorodumi
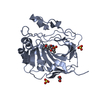
Yorodumi- PDB-1evq: THE CRYSTAL STRUCTURE OF THE THERMOPHILIC CARBOXYLESTERASE EST2 F... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1evq | ||||||
|---|---|---|---|---|---|---|---|
| Title | THE CRYSTAL STRUCTURE OF THE THERMOPHILIC CARBOXYLESTERASE EST2 FROM ALICYCLOBACILLUS ACIDOCALDARIUS | ||||||
 Components Components | SERINE HYDROLASE | ||||||
 Keywords Keywords | HYDROLASE / alpha/beta hydrolase fold | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Alicyclobacillus acidocaldarius (bacteria) Alicyclobacillus acidocaldarius (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MAD / Resolution: 2.6 Å MAD / Resolution: 2.6 Å | ||||||
 Authors Authors | De Simone, G. / Galdiero, S. / Manco, G. / Lang, D. / Rossi, M. / Pedone, C. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2000 Journal: J.Mol.Biol. / Year: 2000Title: A snapshot of a transition state analogue of a novel thermophilic esterase belonging to the subfamily of mammalian hormone-sensitive lipase. Authors: De Simone, G. / Galdiero, S. / Manco, G. / Lang, D. / Rossi, M. / Pedone, C. #1:  Journal: Acta Crystallogr.,Sect.D / Year: 1999 Journal: Acta Crystallogr.,Sect.D / Year: 1999Title: Crystallization and Preliminary X-ray Diffraction Studies of the Carboxylesterase EST2 from Alicyclobacillus acidocaldarius Authors: De Simone, G. / Manco, G. / Galdiero, S. / Lombardi, A. / Rossi, M. / Pavone, V. #2:  Journal: Nat.Struct.Biol. / Year: 1999 Journal: Nat.Struct.Biol. / Year: 1999Title: Crystal Structure of Brefeldin A Esterase, a Bacterial Homolog of the Mammalian Hormone-sensitive Lipase Authors: Wei, Y. / Contreras, J.A. / Sheffield, P. / Osterlund, T. / Derewenda, U. / Kneusel, R.E. / Matern, U. / Holm, C. / Derewenda, Z.S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1evq.cif.gz 1evq.cif.gz | 71 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1evq.ent.gz pdb1evq.ent.gz | 52.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1evq.json.gz 1evq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ev/1evq https://data.pdbj.org/pub/pdb/validation_reports/ev/1evq ftp://data.pdbj.org/pub/pdb/validation_reports/ev/1evq ftp://data.pdbj.org/pub/pdb/validation_reports/ev/1evq | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
| ||||||||
| Details | The biological assembly is a monomer |
- Components
Components
| #1: Protein | Mass: 34526.551 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Alicyclobacillus acidocaldarius (bacteria) Alicyclobacillus acidocaldarius (bacteria)Plasmid: PT7-7SCII / Species (production host): Escherichia coli / Production host:  |
|---|---|
| #2: Chemical | ChemComp-EPE / |
| #3: Chemical | ChemComp-TRS / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.43 Å3/Da / Density % sol: 48.8 % Description: Wavelength 0.9785 is the peak wavelength, 0.9786 is the inflection, and 0.9810 is remote. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 7.8 Details: 2% PEG 400, 100mM Hepes pH 7.8, 2M Ammonium Sulphate, 1mM DTT, VAPOR DIFFUSION, HANGING DROP, temperature 22K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 8.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: X31 / Wavelength: 0.9785, 0.9786, 0.9810 / Beamline: X31 / Wavelength: 0.9785, 0.9786, 0.9810 | ||||||||||||
| Detector | Type: MARRESEARCH / Detector: AREA DETECTOR / Date: Mar 10, 1999 | ||||||||||||
| Radiation | Protocol: MAD / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||
| Radiation wavelength |
| ||||||||||||
| Reflection | Resolution: 2.6→100 Å / Num. all: 203297 / Num. obs: 10886 / % possible obs: 99.4 % / Redundancy: 18 % / Rmerge(I) obs: 0.058 / Net I/σ(I): 31 | ||||||||||||
| Reflection shell | Resolution: 2.6→2.69 Å / Rmerge(I) obs: 0.236 / Num. unique all: 1057 / % possible all: 100 | ||||||||||||
| Reflection shell | *PLUS % possible obs: 100 % / Mean I/σ(I) obs: 8.8 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MAD / Resolution: 2.6→20 Å / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber MAD / Resolution: 2.6→20 Å / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.6→20 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Classification: refinement | |||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.6 Å / Lowest resolution: 20 Å / σ(F): 0 / % reflection Rfree: 5 % / Rfactor obs: 0.216 | |||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: c_angle_deg / Dev ideal: 1.6 |
 Movie
Movie Controller
Controller











 PDBj
PDBj




