[English] 日本語
 Yorodumi
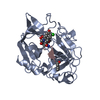
Yorodumi- PDB-1b0e: CRYSTAL STRUCTURE OF PORCINE PANCREATIC ELASTASE WITH MDL 101,146 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1b0e | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF PORCINE PANCREATIC ELASTASE WITH MDL 101,146 | ||||||
 Components Components | PROTEIN (ELASTASE) | ||||||
 Keywords Keywords | HYDROLASE / SERINE PROTEASE / FLUOROETHYL KETONES | ||||||
| Function / homology |  Function and homology information Function and homology informationpancreatic elastase / serine-type endopeptidase activity / proteolysis / extracellular space / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Schreuder, H.A. / Metz, W.A. / Peet, N.P. / Pelton, J.T. / Tardif, C. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 1998 Journal: J.Med.Chem. / Year: 1998Title: Inhibition of human neutrophil elastase. 4. Design, synthesis, X-ray crystallographic analysis, and structure-activity relationships for a series of P2-modified, orally active peptidyl pentafluoroethyl ketones. Authors: Cregge, R.J. / Durham, S.L. / Farr, R.A. / Gallion, S.L. / Hare, C.M. / Hoffman, R.V. / Janusz, M.J. / Kim, H.O. / Koehl, J.R. / Mehdi, S. / Metz, W.A. / Peet, N.P. / Pelton, J.T. / ...Authors: Cregge, R.J. / Durham, S.L. / Farr, R.A. / Gallion, S.L. / Hare, C.M. / Hoffman, R.V. / Janusz, M.J. / Kim, H.O. / Koehl, J.R. / Mehdi, S. / Metz, W.A. / Peet, N.P. / Pelton, J.T. / Schreuder, H.A. / Sunder, S. / Tardif, C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1b0e.cif.gz 1b0e.cif.gz | 65.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1b0e.ent.gz pdb1b0e.ent.gz | 47 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1b0e.json.gz 1b0e.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/b0/1b0e https://data.pdbj.org/pub/pdb/validation_reports/b0/1b0e ftp://data.pdbj.org/pub/pdb/validation_reports/b0/1b0e ftp://data.pdbj.org/pub/pdb/validation_reports/b0/1b0e | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1b0fC  3estS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 25928.031 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #2: Chemical | ChemComp-CA / |
| #3: Chemical | ChemComp-SEI / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.7 Å3/Da / Density % sol: 54.71 % | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Method: vapor diffusion, sitting drop / pH: 5 Details: SITTING DROP PROCEDURE. PROTEIN DROP: 13 MG/ML PPE (SERVA) 9.3 MM SODIUM ACETATE (PH 5.0), 22.4 MM SODIUM SULFATE, 2.2 MM MDL 103,139 IN DMF RESERVOIR: 10 MM SODIUM ACETATE (PH 5.0), 20 MM ...Details: SITTING DROP PROCEDURE. PROTEIN DROP: 13 MG/ML PPE (SERVA) 9.3 MM SODIUM ACETATE (PH 5.0), 22.4 MM SODIUM SULFATE, 2.2 MM MDL 103,139 IN DMF RESERVOIR: 10 MM SODIUM ACETATE (PH 5.0), 20 MM SODIUM SULFATE., vapor diffusion - sitting drop | |||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, sitting drop | |||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 287 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: SIEMENS / Wavelength: 1.5418 ROTATING ANODE / Type: SIEMENS / Wavelength: 1.5418 |
| Detector | Type: SIEMENS / Detector: AREA DETECTOR / Date: Oct 19, 1993 / Details: COLLIMATOR |
| Radiation | Monochromator: GRAPHITE / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→20 Å / Num. obs: 24302 / % possible obs: 93.4 % / Redundancy: 4.2 % / Rsym value: 0.5 |
| Reflection shell | Resolution: 1.8→1.9 Å / Redundancy: 2.4 % / Rsym value: 0.292 / % possible all: 83.2 |
| Reflection | *PLUS Num. measured all: 101240 / Rmerge(I) obs: 0.05 |
| Reflection shell | *PLUS % possible obs: 83.2 % / Rmerge(I) obs: 0.292 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 3EST Resolution: 1.8→8 Å / Isotropic thermal model: RESTRAINED / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 21.4 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.8→1.88 Å / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.1 / Classification: refinement X-PLOR / Version: 3.1 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller










 PDBj
PDBj