[English] 日本語
 Yorodumi
Yorodumi- PDB-6shi: Human kallikrein 7 with aromatic coumarinic ester compound 2 cova... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6shi | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human kallikrein 7 with aromatic coumarinic ester compound 2 covalently bound to H57 | ||||||
 Components Components | Kallikrein-7 | ||||||
 Keywords Keywords | HYDROLASE / Serine Protease / Covalent Inhibitor / Complex | ||||||
| Function / homology |  Function and homology information Function and homology informationstratum corneum chymotryptic enzyme / positive regulation of antibacterial peptide production / epidermal lamellar body / cornified envelope / Differentiation of Keratinocytes in Interfollicular Epidermis in Mammalian Skin / extracellular matrix disassembly / epidermis development / Degradation of the extracellular matrix / serine-type peptidase activity / secretory granule ...stratum corneum chymotryptic enzyme / positive regulation of antibacterial peptide production / epidermal lamellar body / cornified envelope / Differentiation of Keratinocytes in Interfollicular Epidermis in Mammalian Skin / extracellular matrix disassembly / epidermis development / Degradation of the extracellular matrix / serine-type peptidase activity / secretory granule / protein maturation / metalloendopeptidase activity / peptidase activity / serine-type endopeptidase activity / proteolysis / extracellular space / extracellular region Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.85 Å MOLECULAR REPLACEMENT / Resolution: 1.85 Å | ||||||
 Authors Authors | Hanke, S. / Straeter, N. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2020 Journal: J.Med.Chem. / Year: 2020Title: Structural Studies on the Inhibitory Binding Mode of Aromatic Coumarinic Esters to Human Kallikrein-Related Peptidase 7. Authors: Hanke, S. / Tindall, C.A. / Pippel, J. / Ulbricht, D. / Pirotte, B. / Reboud-Ravaux, M. / Heiker, J.T. / Strater, N. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6shi.cif.gz 6shi.cif.gz | 764.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6shi.ent.gz pdb6shi.ent.gz | 633 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6shi.json.gz 6shi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sh/6shi https://data.pdbj.org/pub/pdb/validation_reports/sh/6shi ftp://data.pdbj.org/pub/pdb/validation_reports/sh/6shi ftp://data.pdbj.org/pub/pdb/validation_reports/sh/6shi | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6shhC  6sjuC  6y4sC  2qxiS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
- Components
Components
-Protein , 1 types, 8 molecules ABCDEFGH
| #1: Protein | Mass: 24481.160 Da / Num. of mol.: 8 Source method: isolated from a genetically manipulated source Details: The inhibitor is covalently attached to H57 / Source: (gene. exp.)  Homo sapiens (human) / Gene: KLK7, PRSS6, SCCE / Production host: Homo sapiens (human) / Gene: KLK7, PRSS6, SCCE / Production host:  References: UniProt: P49862, stratum corneum chymotryptic enzyme |
|---|
-Non-polymers , 5 types, 1988 molecules 
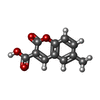







| #2: Chemical | ChemComp-PGE / #3: Chemical | ChemComp-SH8 / #4: Chemical | ChemComp-SO4 / #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.65 Å3/Da / Density % sol: 53.65 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 8.5 Details: 2.9 M ammonium sulphate, 0.1 M HEPES, pH 8.5, 0.5- 2 % PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  BESSY BESSY  / Beamline: 14.1 / Wavelength: 0.920172 Å / Beamline: 14.1 / Wavelength: 0.920172 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Mar 28, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.920172 Å / Relative weight: 1 |
| Reflection | Resolution: 1.85→47.08 Å / Num. obs: 177629 / % possible obs: 99.8 % / Redundancy: 7.4 % / Biso Wilson estimate: 26.18 Å2 / CC1/2: 0.997 / Rmerge(I) obs: 0.146 / Rrim(I) all: 0.169 / Χ2: 1.01 / Net I/σ(I): 9.5 |
| Reflection shell | Resolution: 1.85→1.88 Å / Redundancy: 7.3 % / Rmerge(I) obs: 1.823 / Mean I/σ(I) obs: 1.1 / Num. unique obs: 8592 / CC1/2: 0.423 / Rrim(I) all: 2.119 / Χ2: 1.01 / % possible all: 98.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2qxi Resolution: 1.85→46.6 Å / Cor.coef. Fo:Fc: 0.952 / Cor.coef. Fo:Fc free: 0.937 / SU R Cruickshank DPI: 0.123 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.137 / SU Rfree Blow DPI: 0.123 / SU Rfree Cruickshank DPI: 0.116
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 204.2 Å2 / Biso mean: 33.78 Å2 / Biso min: 13.36 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.21 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.85→46.6 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.85→1.86 Å / Rfactor Rfree error: 0 / Total num. of bins used: 50
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj