+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9644 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
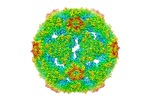
| Title | Cryo-EM structure of CVA10 empty particle | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | picornavirus uncoating / receptor binding / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / virion component / ribonucleoside triphosphate phosphatase activity / nucleoside-triphosphate phosphatase ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / virion component / ribonucleoside triphosphate phosphatase activity / nucleoside-triphosphate phosphatase / host cell / channel activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / symbiont entry into host cell / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |   Coxsackievirus A10 Coxsackievirus A10 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Zhu L / Sun Y | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2018 Journal: Nat Commun / Year: 2018Title: Structures of Coxsackievirus A10 unveil the molecular mechanisms of receptor binding and viral uncoating. Authors: Ling Zhu / Yao Sun / Jinyan Fan / Bin Zhu / Lei Cao / Qiang Gao / Yanjun Zhang / Hongrong Liu / Zihe Rao / Xiangxi Wang /  Abstract: Coxsackievirus A10 (CVA10), a human type-A Enterovirus (HEV-A), can cause diseases ranging from hand-foot-and-mouth disease to polio-myelitis-like disease. CVA10, together with some other HEV-As, ...Coxsackievirus A10 (CVA10), a human type-A Enterovirus (HEV-A), can cause diseases ranging from hand-foot-and-mouth disease to polio-myelitis-like disease. CVA10, together with some other HEV-As, utilizing the molecule KREMEN1 as an entry receptor, constitutes a KREMEN1-dependent subgroup within HEV-As. Currently, there is no vaccine or antiviral therapy available for treating diseases caused by CVA10. The atomic-resolution structure of the CVA10 virion, which is within the KREMEN1-dependent subgroup, shows significant conformational differences in the putative receptor binding sites and serotype-specific epitopes, when compared to the SCARB2-dependent subgroup of HEV-A, such as EV71, highlighting specific differences between the sub-groups. We also report two expanded structures of CVA10, an empty particle and uncoating intermediate at atomic resolution, as well as a medium-resolution genome structure reconstructed using a symmetry-mismatch method. Structural comparisons coupled with previous results, reveal an ordered signal transmission process for enterovirus uncoating, converting exo-genetic receptor-attachment inputs into a generic RNA release mechanism. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9644.map.gz emd_9644.map.gz | 167 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9644-v30.xml emd-9644-v30.xml emd-9644.xml emd-9644.xml | 13.3 KB 13.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9644.png emd_9644.png | 267.4 KB | ||
| Filedesc metadata |  emd-9644.cif.gz emd-9644.cif.gz | 6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9644 http://ftp.pdbj.org/pub/emdb/structures/EMD-9644 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9644 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9644 | HTTPS FTP |
-Related structure data
| Related structure data |  6akuMC  9642C  9643C  9652C  6aksC  6aktC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9644.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9644.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Coxsackievirus A10
| Entire | Name:   Coxsackievirus A10 Coxsackievirus A10 |
|---|---|
| Components |
|
-Supramolecule #1: Coxsackievirus A10
| Supramolecule | Name: Coxsackievirus A10 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 42769 / Sci species name: Coxsackievirus A10 / Virus type: VIRION / Virus isolate: SEROTYPE / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: VP1
| Macromolecule | Name: VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Coxsackievirus A10 Coxsackievirus A10 |
| Molecular weight | Theoretical: 33.232383 KDa |
| Recombinant expression | Organism:  Chlorocebus aethiops (grivet) Chlorocebus aethiops (grivet) |
| Sequence | String: GDPVEDIIHD ALGSTARRAI SSATNVESAA NTTPSSHRLE TGRVPALQAA ETGATSNATD ENMIETRCVV NRNGVLETTI NHFFSRSGL VGVVNLTDGG TDTTGYVTWD IDIMGFVQLR RKCEMFTYMR FNAEFTFVTT TKNGEARPYM LQYMYVPPGA P KPTGRDAF ...String: GDPVEDIIHD ALGSTARRAI SSATNVESAA NTTPSSHRLE TGRVPALQAA ETGATSNATD ENMIETRCVV NRNGVLETTI NHFFSRSGL VGVVNLTDGG TDTTGYVTWD IDIMGFVQLR RKCEMFTYMR FNAEFTFVTT TKNGEARPYM LQYMYVPPGA P KPTGRDAF QWQTATNPSV FVKLTDPPAQ VSVPFMSPAS AYQWFYDGYP TFGQHPETSN TTYGLCPNNM MGTFAVRVVS RE ASQLKLQ TRVYMKLKHV RAWVPRPIRS QPYLLKNFPN YDSSKITNSA RDRSSIKQAN M UniProtKB: Genome polyprotein |
-Macromolecule #2: VP2
| Macromolecule | Name: VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Coxsackievirus A10 Coxsackievirus A10 |
| Molecular weight | Theoretical: 27.783105 KDa |
| Recombinant expression | Organism:  Chlorocebus aethiops (grivet) Chlorocebus aethiops (grivet) |
| Sequence | String: SPSVEACGYS DRVAQLTVGN SSITTQEAAN IVLAYGEWPE YCPDTDATAV DKPTRPDVSV NRFYTLDSKM WQENSTGWYW KFPDVLNKT GVFGQNAQFH YLYRSGFCLH VQCNASKFHQ GALLVAVIPE FVIAGRGSNT KPNEAPHPGF TTTFPGTTGA T FHDPYVLD ...String: SPSVEACGYS DRVAQLTVGN SSITTQEAAN IVLAYGEWPE YCPDTDATAV DKPTRPDVSV NRFYTLDSKM WQENSTGWYW KFPDVLNKT GVFGQNAQFH YLYRSGFCLH VQCNASKFHQ GALLVAVIPE FVIAGRGSNT KPNEAPHPGF TTTFPGTTGA T FHDPYVLD SGVPLSQALI YPHQWINLRT NNCATVIVPY INAVPFDSAI NHSNFGLIVI PVSPLKYSSG ATTAIPITIT IA PLNSEFG GLRQAVSQ UniProtKB: Genome polyprotein |
-Macromolecule #3: VP3
| Macromolecule | Name: VP3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Coxsackievirus A10 Coxsackievirus A10 |
| Molecular weight | Theoretical: 26.279826 KDa |
| Recombinant expression | Organism:  Chlorocebus aethiops (grivet) Chlorocebus aethiops (grivet) |
| Sequence | String: GIPAELRPGT NQFLTTDDDT AAPILPGFTP TPTIHIPGEV HSLLELCRVE TILEVNNTTE ATGLTRLLIP VSSQNKADEL CAAFMVDPG RIGPWQSTLV GQICRYYTQW SGSLKVTFMF TGSFMATGKM LVAYSPPGSA QPANRETAML GTHVIWDFGL Q SSVSLVIP ...String: GIPAELRPGT NQFLTTDDDT AAPILPGFTP TPTIHIPGEV HSLLELCRVE TILEVNNTTE ATGLTRLLIP VSSQNKADEL CAAFMVDPG RIGPWQSTLV GQICRYYTQW SGSLKVTFMF TGSFMATGKM LVAYSPPGSA QPANRETAML GTHVIWDFGL Q SSVSLVIP WISNTHFRTA KTGGNYDYYT AGVVTLWYQT NYVVPPETPG EAYIIAMGAD LYKFTLKICK DTDEVTQQAV LQ UniProtKB: Genome polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















