[English] 日本語
 Yorodumi
Yorodumi- EMDB-20275: Poliovirus 135S-like expanded particle in complex with a monoclon... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20275 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Poliovirus 135S-like expanded particle in complex with a monoclonal antibody directed against the N-terminal extension of capsid protein VP1 | |||||||||
 Map data Map data | Sharpened Poliovirus 135S-like expanded particle in complex with a monoclonal antibody directed against the N-terminal extension of capsid protein VP1 | |||||||||
 Sample Sample | Human poliovirus 1 Mahoney != Poliovirus type 1 (strain Mahoney) Human poliovirus 1 Mahoney
| |||||||||
 Keywords Keywords | Virus-antibody complex / poliovirus / cell-entry intermediate / expanded virus / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host translation initiation / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane ...symbiont-mediated suppression of host translation initiation / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |   Poliovirus type 1 (strain Mahoney) Poliovirus type 1 (strain Mahoney) | |||||||||
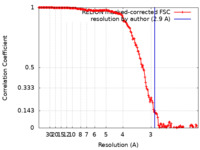
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Hogle JM / Filman DJ | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: PLoS Pathog / Year: 2020 Journal: PLoS Pathog / Year: 2020Title: Cryo-EM structures reveal two distinct conformational states in a picornavirus cell entry intermediate. Authors: Pranav N M Shah / David J Filman / Krishanthi S Karunatilaka / Emma L Hesketh / Elisabetta Groppelli / Mike Strauss / James M Hogle /    Abstract: The virions of enteroviruses such as poliovirus undergo a global conformational change after binding to the cellular receptor, characterized by a 4% expansion, and by the opening of holes at the two ...The virions of enteroviruses such as poliovirus undergo a global conformational change after binding to the cellular receptor, characterized by a 4% expansion, and by the opening of holes at the two and quasi-three-fold symmetry axes of the capsid. The resultant particle is called a 135S particle or A-particle and is thought to be on the pathway to a productive infection. Previously published studies have concluded that the membrane-interactive peptides, namely VP4 and the N-terminus of VP1, are irreversibly externalized in the 135S particle. However, using established protocols to produce the 135S particle, and single particle cryo-electron microscopy methods, we have identified at least two unique states that we call the early and late 135S particle. Surprisingly, only in the "late" 135S particles have detectable levels of the VP1 N-terminus been trapped outside the capsid. Moreover, we observe a distinct density inside the capsid that can be accounted for by VP4 that remains associated with the genome. Taken together our results conclusively demonstrate that the 135S particle is not a unique conformation, but rather a family of conformations that could exist simultaneously. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20275.map.gz emd_20275.map.gz | 321.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20275-v30.xml emd-20275-v30.xml emd-20275.xml emd-20275.xml | 15.4 KB 15.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20275_fsc.xml emd_20275_fsc.xml | 15.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_20275.png emd_20275.png | 197.5 KB | ||
| Masks |  emd_20275_msk_1.map emd_20275_msk_1.map | 347.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-20275.cif.gz emd-20275.cif.gz | 6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20275 http://ftp.pdbj.org/pub/emdb/structures/EMD-20275 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20275 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20275 | HTTPS FTP |
-Related structure data
| Related structure data |  6p9oMC  6p9wC  6pszC  6q0bC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20275.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20275.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened Poliovirus 135S-like expanded particle in complex with a monoclonal antibody directed against the N-terminal extension of capsid protein VP1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.056 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_20275_msk_1.map emd_20275_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human poliovirus 1 Mahoney
| Entire | Name:   Human poliovirus 1 Mahoney Human poliovirus 1 Mahoney |
|---|---|
| Components |
|
-Supramolecule #1: Poliovirus type 1 (strain Mahoney)
| Supramolecule | Name: Poliovirus type 1 (strain Mahoney) / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 12081 / Sci species name: Poliovirus type 1 (strain Mahoney) / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 10.0 MDa |
| Virus shell | Shell ID: 1 / Name: Capsid / Diameter: 300.0 Å / T number (triangulation number): 1 |
-Macromolecule #1: VP1
| Macromolecule | Name: VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Poliovirus type 1 (strain Mahoney) / Strain: Mahoney Poliovirus type 1 (strain Mahoney) / Strain: Mahoney |
| Molecular weight | Theoretical: 33.488613 KDa |
| Sequence | String: GLGQMLESMI DNTVRETVGA ATSRDALPNT EASGPTHSKE IPALTAVETG ATNPLVPSDT VQTRHVVQHR SRSESSIESF FARGACVTI MTVDNPASTT NKDKLFAVWK ITYKDTVQLR RKLEFFTYSR FDMELTFVVT ANFTETNNGH ALNQVYQIMY V PPGAPVPE ...String: GLGQMLESMI DNTVRETVGA ATSRDALPNT EASGPTHSKE IPALTAVETG ATNPLVPSDT VQTRHVVQHR SRSESSIESF FARGACVTI MTVDNPASTT NKDKLFAVWK ITYKDTVQLR RKLEFFTYSR FDMELTFVVT ANFTETNNGH ALNQVYQIMY V PPGAPVPE KWDDYTWQTS SNPSIFYTYG TAPARISVPY VGISNAYSHF YDGFSKVPLK DQSAALGDSL YGAASLNDFG IL AVRVVND HNPTKVTSKI RVYLKPKHIR VWCPRPPRAV AYYGPGVDYK DGTLTPLSTK DLTTY UniProtKB: Genome polyprotein |
-Macromolecule #2: VP2
| Macromolecule | Name: VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Poliovirus type 1 (strain Mahoney) / Strain: Mahoney Poliovirus type 1 (strain Mahoney) / Strain: Mahoney |
| Molecular weight | Theoretical: 30.075783 KDa |
| Sequence | String: SPNIEACGYS DRVLQLTLGN STITTQEAAN SVVAYGRWPE YLRDSEANPV DQPTEPDVAA CRFYTLDTVS WTKESRGWWW KLPDALRDM GLFGQNMYYH YLGRSGYTVH VQCNASKFHQ GALGVFAVPE MCLAGDSNTT TMHTSYQNAN PGEKGGTFTG T FTPDNNQT ...String: SPNIEACGYS DRVLQLTLGN STITTQEAAN SVVAYGRWPE YLRDSEANPV DQPTEPDVAA CRFYTLDTVS WTKESRGWWW KLPDALRDM GLFGQNMYYH YLGRSGYTVH VQCNASKFHQ GALGVFAVPE MCLAGDSNTT TMHTSYQNAN PGEKGGTFTG T FTPDNNQT SPARRFCPVD YLLGNGTLLG NAFVFPHQII NLRTNNCATL VLPYVNSLSI DSMVKHNNWG IAILPLAPLN FA SESSPEI PITLTIAPMC CEFNGLRNIT LPRLQ UniProtKB: Genome polyprotein |
-Macromolecule #3: VP3
| Macromolecule | Name: VP3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Poliovirus type 1 (strain Mahoney) / Strain: Mahoney Poliovirus type 1 (strain Mahoney) / Strain: Mahoney |
| Molecular weight | Theoretical: 26.547482 KDa |
| Sequence | String: GLPVMNTPGS NQYLTADNFQ SPCALPEFDV TPPIDIPGEV KNMMELAEID TMIPFDLSAT KKNTMEMYRV RLSDKPHTDD PILCLSLSP ASDPRLSHTM LGEILNYYTH WAGSLKFTFL FCGSMMATGK LLVSYAPPGA DPPKKRKEAM LGTHVIWDIG L QSSCTMVV ...String: GLPVMNTPGS NQYLTADNFQ SPCALPEFDV TPPIDIPGEV KNMMELAEID TMIPFDLSAT KKNTMEMYRV RLSDKPHTDD PILCLSLSP ASDPRLSHTM LGEILNYYTH WAGSLKFTFL FCGSMMATGK LLVSYAPPGA DPPKKRKEAM LGTHVIWDIG L QSSCTMVV PWISNTTYRQ TIDDSFTEGG YISVFYQTRI VVPLSTPREM DILGFVSACN DFSVRLLRDT THIEQKALAQ UniProtKB: Genome polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: phosphate-buffered saline |
| Grid | Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: LACEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Details: unspecified |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK III |
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 2 / Average electron dose: 2.17 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-6p9o: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)