[English] 日本語
 Yorodumi
Yorodumi- EMDB-8621: The cryo-EM structure of YjeQ bound to the 30S subunit suggests a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8621 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The cryo-EM structure of YjeQ bound to the 30S subunit suggests a fidelity checkpoint function for this protein in ribosome assembly | |||||||||
 Map data Map data | Structure of the mature 30S subunit in complex with YjeQ (RsgA) GTPase | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ribosome assembly / 30S subunit / YjeQ protein / RsgA protein / RIBOSOME-Hydrolase complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationguanosine tetraphosphate binding / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / four-way junction DNA binding / negative regulation of translational initiation / mRNA regulatory element binding translation repressor activity / regulation of DNA-templated transcription elongation / transcription elongation factor complex / transcription antitermination ...guanosine tetraphosphate binding / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / four-way junction DNA binding / negative regulation of translational initiation / mRNA regulatory element binding translation repressor activity / regulation of DNA-templated transcription elongation / transcription elongation factor complex / transcription antitermination / DNA endonuclease activity / DNA-templated transcription termination / maintenance of translational fidelity / mRNA 5'-UTR binding / GDP binding / regulation of translation / ribosome biogenesis / ribosomal small subunit biogenesis / ribosomal small subunit assembly / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / response to antibiotic / mRNA binding / GTPase activity / GTP binding / RNA binding / zinc ion binding / metal ion binding / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
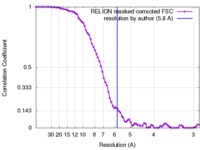
| Method | single particle reconstruction / cryo EM / Resolution: 5.8 Å | |||||||||
 Authors Authors | Razi A / Guarne A | |||||||||
| Funding support |  Canada, 1 items Canada, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2017 Journal: Proc Natl Acad Sci U S A / Year: 2017Title: The cryo-EM structure of YjeQ bound to the 30S subunit suggests a fidelity checkpoint function for this protein in ribosome assembly. Authors: Aida Razi / Alba Guarné / Joaquin Ortega /  Abstract: Recent work suggests that bacterial YjeQ (RsgA) participates in the late stages of assembly of the 30S subunit and aids the assembly of the decoding center but also binds the mature 30S subunit with ...Recent work suggests that bacterial YjeQ (RsgA) participates in the late stages of assembly of the 30S subunit and aids the assembly of the decoding center but also binds the mature 30S subunit with high affinity. To determine the function and mechanisms of YjeQ in the context of the mature subunit, we determined the cryo-EM structure of the fully assembled 30S subunit in complex with YjeQ at 5.8-Å resolution. We found that binding of YjeQ stabilizes helix 44 into a conformation similar to that adopted by the subunit during proofreading. This finding indicates that, along with acting as an assembly factor, YjeQ has a role as a checkpoint protein, consisting of testing the proofreading ability of the 30S subunit. The structure also informs the mechanism by which YjeQ implements the release from the 30S subunit of a second assembly factor, called RbfA. Finally, it reveals how the 30S subunit stimulates YjeQ GTPase activity and leads to release of the protein. Checkpoint functions have been described for eukaryotic ribosome assembly factors; however, this work describes an example of a bacterial assembly factor that tests a specific translation mechanism of the 30S subunit. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8621.map.gz emd_8621.map.gz | 4.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8621-v30.xml emd-8621-v30.xml emd-8621.xml emd-8621.xml | 34.5 KB 34.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8621_fsc.xml emd_8621_fsc.xml | 7.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_8621.png emd_8621.png | 46.4 KB | ||
| Filedesc metadata |  emd-8621.cif.gz emd-8621.cif.gz | 9.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8621 http://ftp.pdbj.org/pub/emdb/structures/EMD-8621 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8621 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8621 | HTTPS FTP |
-Validation report
| Summary document |  emd_8621_validation.pdf.gz emd_8621_validation.pdf.gz | 415.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8621_full_validation.pdf.gz emd_8621_full_validation.pdf.gz | 414.8 KB | Display | |
| Data in XML |  emd_8621_validation.xml.gz emd_8621_validation.xml.gz | 10.2 KB | Display | |
| Data in CIF |  emd_8621_validation.cif.gz emd_8621_validation.cif.gz | 13.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8621 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8621 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8621 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8621 | HTTPS FTP |
-Related structure data
| Related structure data |  5uz4MC  8626C  8627C  8628C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8621.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8621.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of the mature 30S subunit in complex with YjeQ (RsgA) GTPase | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.45 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Structure of the 30S subunit in complex with YjeQ GTPase
+Supramolecule #1: Structure of the 30S subunit in complex with YjeQ GTPase
+Macromolecule #1: 16S RIBOSOMAL RNA
+Macromolecule #2: 30S ribosomal protein S3
+Macromolecule #3: 30S ribosomal protein S4
+Macromolecule #4: 30S ribosomal protein S5
+Macromolecule #5: 30S ribosomal protein S6
+Macromolecule #6: 30S ribosomal protein S7
+Macromolecule #7: 30S ribosomal protein S8
+Macromolecule #8: 30S ribosomal protein S9
+Macromolecule #9: 30S ribosomal protein S10
+Macromolecule #10: 30S ribosomal protein S11
+Macromolecule #11: 30S ribosomal protein S12
+Macromolecule #12: 30S ribosomal protein S13
+Macromolecule #13: 30S ribosomal protein S14
+Macromolecule #14: 30S ribosomal protein S15
+Macromolecule #15: 30S ribosomal protein S16
+Macromolecule #16: 30S ribosomal protein S17
+Macromolecule #17: 30S ribosomal protein S18
+Macromolecule #18: 30S ribosomal protein S19
+Macromolecule #19: 30S ribosomal protein S20
+Macromolecule #20: 30S ribosomal protein S2
+Macromolecule #21: Small ribosomal subunit biogenesis GTPase RsgA
+Macromolecule #22: ZINC ION
+Macromolecule #23: 3'-O-(N-methylanthraniloyl)-beta:gamma-imidoguanosine-5'-triphosphate
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: C-flat CFT-222C / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-20 / Average exposure time: 0.5 sec. / Average electron dose: 1.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 34482 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 25000 |
| Sample stage | Specimen holder model: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: OTHER |
|---|---|
| Output model |  PDB-5uz4: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)