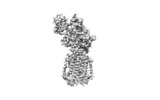
登録情報 データベース : EMDB / ID : EMD-7796タイトル Structure of human Patched1 in complex with native Sonic Hedgehog A protein structure 複合体 : Ptcタンパク質・ペプチド : Protein patched homolog 1タンパク質・ペプチド : Sonic hedgehog proteinリガンド : N-ACETYL-D-GLUCOSAMINEリガンド : ZINC IONリガンド : PALMITIC ACID機能・相同性 分子機能 ドメイン・相同性 構成要素
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Homo sapiens (ヒト)手法 / 解像度 : 3.5 Å Qi X / Li X ジャーナル : Nature / 年 : 2018タイトル : Structures of human Patched and its complex with native palmitoylated sonic hedgehog.著者 : Xiaofeng Qi / Philip Schmiege / Elias Coutavas / Jiawei Wang / Xiaochun Li / 要旨 : Hedgehog (HH) signalling governs embryogenesis and adult tissue homeostasis in mammals and other multicellular organisms. Whereas deficient HH signalling leads to birth defects, unrestrained HH ... Hedgehog (HH) signalling governs embryogenesis and adult tissue homeostasis in mammals and other multicellular organisms. Whereas deficient HH signalling leads to birth defects, unrestrained HH signalling is implicated in human cancers. N-terminally palmitoylated HH releases the repression of Patched to the oncoprotein smoothened (SMO); however, the mechanism by which HH recognizes Patched is unclear. Here we report cryo-electron microscopy structures of human patched 1 (PTCH1) alone and in complex with the N-terminal domain of 'native' sonic hedgehog (native SHH-N has both a C-terminal cholesterol and an N-terminal fatty-acid modification), at resolutions of 3.5 Å and 3.8 Å, respectively. The structure of PTCH1 has internal two-fold pseudosymmetry in the transmembrane core, which features a sterol-sensing domain and two homologous extracellular domains, resembling the architecture of Niemann-Pick C1 (NPC1) protein. The palmitoylated N terminus of SHH-N inserts into a cavity between the extracellular domains of PTCH1 and dominates the PTCH1-SHH-N interface, which is distinct from that reported for SHH-N co-receptors. Our biochemical assays show that SHH-N may use another interface, one that is required for its co-receptor binding, to recruit PTCH1 in the absence of a covalently attached palmitate. Our work provides atomic insights into the recognition of the N-terminal domain of HH (HH-N) by PTCH1, offers a structural basis for cooperative binding of HH-N to various receptors and serves as a molecular framework for HH signalling and its malfunction in disease. 履歴 登録 2018年4月18日 - ヘッダ(付随情報) 公開 2018年7月4日 - マップ公開 2018年7月11日 - 更新 2019年4月17日 - 現状 2019年4月17日 処理サイト : RCSB / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報 マップデータ
マップデータ 試料
試料 機能・相同性情報
機能・相同性情報 Homo sapiens (ヒト)
Homo sapiens (ヒト) データ登録者
データ登録者 引用
引用 ジャーナル: Nature / 年: 2018
ジャーナル: Nature / 年: 2018

 構造の表示
構造の表示 ムービービューア
ムービービューア SurfView
SurfView Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク emd_7796.map.gz
emd_7796.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-7796-v30.xml
emd-7796-v30.xml emd-7796.xml
emd-7796.xml EMDBヘッダ
EMDBヘッダ emd_7796.png
emd_7796.png http://ftp.pdbj.org/pub/emdb/structures/EMD-7796
http://ftp.pdbj.org/pub/emdb/structures/EMD-7796 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7796
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7796 emd_7796_validation.pdf.gz
emd_7796_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_7796_full_validation.pdf.gz
emd_7796_full_validation.pdf.gz emd_7796_validation.xml.gz
emd_7796_validation.xml.gz emd_7796_validation.cif.gz
emd_7796_validation.cif.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7796
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7796 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7796
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7796 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_7796.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_7796.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素 Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト)
 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 画像解析
画像解析 ムービー
ムービー コントローラー
コントローラー





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)