登録情報 データベース : EMDB / ID : EMD-7769タイトル Cryo-EM reconstruction of synthetic tau: four tandem repeats of first repeat (R1) sequence, bound to the microtubule Cryo-EM reconstructions of synthetic tau bound to microtubules. Construct contains the first repeat sequence repeated four times. 複合体 : Ternary complex of alpha-beta tubulin with synthetic (R1x4) tau複合体 : Tubulin alpha-1B chainタンパク質・ペプチド : Tubulin alpha-1B chain複合体 : Tubulin beta chainタンパク質・ペプチド : Tubulin beta chain複合体 : Microtubule-associated protein tauタンパク質・ペプチド : Microtubule-associated protein tauリガンド : GUANOSINE-5'-TRIPHOSPHATEリガンド : MAGNESIUM IONリガンド : GUANOSINE-5'-DIPHOSPHATE / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Sus scrofa (ブタ) / Homo sapiens (ヒト)手法 / / 解像度 : 3.2 Å Nogales E / Kellogg EH 資金援助 Organization Grant number 国 National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) 051487
ジャーナル : Science / 年 : 2018タイトル : Near-atomic model of microtubule-tau interactions.著者 : Elizabeth H Kellogg / Nisreen M A Hejab / Simon Poepsel / Kenneth H Downing / Frank DiMaio / Eva Nogales / 要旨 : Tau is a developmentally regulated axonal protein that stabilizes and bundles microtubules (MTs). Its hyperphosphorylation is thought to cause detachment from MTs and subsequent aggregation into ... Tau is a developmentally regulated axonal protein that stabilizes and bundles microtubules (MTs). Its hyperphosphorylation is thought to cause detachment from MTs and subsequent aggregation into fibrils implicated in Alzheimer's disease. It is unclear which tau residues are crucial for tau-MT interactions, where tau binds on MTs, and how it stabilizes them. We used cryo-electron microscopy to visualize different tau constructs on MTs and computational approaches to generate atomic models of tau-tubulin interactions. The conserved tubulin-binding repeats within tau adopt similar extended structures along the crest of the protofilament, stabilizing the interface between tubulin dimers. Our structures explain the effect of phosphorylation on MT affinity and lead to a model of tau repeats binding in tandem along protofilaments, tethering together tubulin dimers and stabilizing polymerization interfaces. 履歴 登録 2018年3月28日 - ヘッダ(付随情報) 公開 2018年4月25日 - マップ公開 2018年5月23日 - 更新 2024年3月13日 - 現状 2024年3月13日 処理サイト : RCSB / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報
 Homo sapiens (ヒト)
Homo sapiens (ヒト) データ登録者
データ登録者 米国, 1件
米国, 1件  引用
引用 ジャーナル: Science / 年: 2018
ジャーナル: Science / 年: 2018
 構造の表示
構造の表示 ムービービューア
ムービービューア SurfView
SurfView Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク emd_7769.map.gz
emd_7769.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-7769-v30.xml
emd-7769-v30.xml emd-7769.xml
emd-7769.xml EMDBヘッダ
EMDBヘッダ emd_7769.png
emd_7769.png emd-7769.cif.gz
emd-7769.cif.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-7769
http://ftp.pdbj.org/pub/emdb/structures/EMD-7769 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7769
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7769 emd_7769_validation.pdf.gz
emd_7769_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_7769_full_validation.pdf.gz
emd_7769_full_validation.pdf.gz emd_7769_validation.xml.gz
emd_7769_validation.xml.gz emd_7769_validation.cif.gz
emd_7769_validation.cif.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7769
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7769 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7769
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7769 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
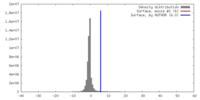
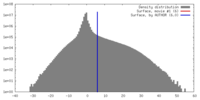
マップ ダウンロード / ファイル: emd_7769.map.gz / 形式: CCP4 / 大きさ: 512 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_7769.map.gz / 形式: CCP4 / 大きさ: 512 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 画像解析
画像解析
 ムービー
ムービー コントローラー
コントローラー





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)