+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8266 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Near-atomic cryo-EM structure of PRC1 bound to the microtubule | ||||||||||||
 Map data Map data | Near-atomic cryo-EM structure of PRC1 bound to the microtubule | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | cytoskeleton / mitosis / microtubule / microtubule-associated protein / STRUCTURAL PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationcontractile ring / mitotic spindle midzone / mitotic spindle elongation / mitotic spindle midzone assembly / RHO GTPases activate CIT / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Resolution of Sister Chromatid Cohesion / Hedgehog 'off' state / Cilium Assembly / Intraflagellar transport ...contractile ring / mitotic spindle midzone / mitotic spindle elongation / mitotic spindle midzone assembly / RHO GTPases activate CIT / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Resolution of Sister Chromatid Cohesion / Hedgehog 'off' state / Cilium Assembly / Intraflagellar transport / COPI-dependent Golgi-to-ER retrograde traffic / Mitotic Prometaphase / Carboxyterminal post-translational modifications of tubulin / RHOH GTPase cycle / EML4 and NUDC in mitotic spindle formation / Sealing of the nuclear envelope (NE) by ESCRT-III / Kinesins / PKR-mediated signaling / Separation of Sister Chromatids / The role of GTSE1 in G2/M progression after G2 checkpoint / Aggrephagy / RHO GTPases activate IQGAPs / RHO GTPases Activate Formins / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / MHC class II antigen presentation / Recruitment of NuMA to mitotic centrosomes / COPI-mediated anterograde transport / kinesin binding / intercellular bridge / regulation of cytokinesis / spindle microtubule / structural constituent of cytoskeleton / microtubule cytoskeleton organization / spindle / neuron migration / spindle pole / mitotic cell cycle / chromosome / microtubule cytoskeleton / midbody / microtubule binding / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / microtubule / cell division / GTPase activity / positive regulation of cell population proliferation / protein kinase binding / GTP binding / nucleoplasm / metal ion binding / identical protein binding / nucleus / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||
 Authors Authors | Kellogg EH / Howes S | ||||||||||||
| Funding support |  United States, United States,  Spain, 3 items Spain, 3 items
| ||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2016 Journal: Proc Natl Acad Sci U S A / Year: 2016Title: Near-atomic cryo-EM structure of PRC1 bound to the microtubule. Authors: Elizabeth H Kellogg / Stuart Howes / Shih-Chieh Ti / Erney Ramírez-Aportela / Tarun M Kapoor / Pablo Chacón / Eva Nogales /   Abstract: Proteins that associate with microtubules (MTs) are crucial to generate MT arrays and establish different cellular architectures. One example is PRC1 (protein regulator of cytokinesis 1), which cross- ...Proteins that associate with microtubules (MTs) are crucial to generate MT arrays and establish different cellular architectures. One example is PRC1 (protein regulator of cytokinesis 1), which cross-links antiparallel MTs and is essential for the completion of mitosis and cytokinesis. Here we describe a 4-Å-resolution cryo-EM structure of monomeric PRC1 bound to MTs. Residues in the spectrin domain of PRC1 contacting the MT are highly conserved and interact with the same pocket recognized by kinesin. We additionally found that PRC1 promotes MT assembly even in the presence of the MT stabilizer taxol. Interestingly, the angle of the spectrin domain on the MT surface corresponds to the previously observed cross-bridge angle between MTs cross-linked by full-length, dimeric PRC1. This finding, together with molecular dynamic simulations describing the intrinsic flexibility of PRC1, suggests that the MT-spectrin domain interface determines the geometry of the MT arrays cross-linked by PRC1. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8266.map.gz emd_8266.map.gz | 482.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8266-v30.xml emd-8266-v30.xml emd-8266.xml emd-8266.xml | 19.9 KB 19.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8266.png emd_8266.png | 407.4 KB | ||
| Filedesc metadata |  emd-8266.cif.gz emd-8266.cif.gz | 7.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8266 http://ftp.pdbj.org/pub/emdb/structures/EMD-8266 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8266 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8266 | HTTPS FTP |
-Related structure data
| Related structure data |  5kmgMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8266.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8266.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Near-atomic cryo-EM structure of PRC1 bound to the microtubule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Ternary complex of PRC1 fragment (PRC1-SC), alpha-tubulin, and be...
| Entire | Name: Ternary complex of PRC1 fragment (PRC1-SC), alpha-tubulin, and beta-tubulin. |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of PRC1 fragment (PRC1-SC), alpha-tubulin, and be...
| Supramolecule | Name: Ternary complex of PRC1 fragment (PRC1-SC), alpha-tubulin, and beta-tubulin. type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 Details: The PRC1 fragment referred to as PRC1-SC contains the spectrin domain and the unstructured C-terminal domain, spanning (337-620) |
|---|
-Macromolecule #1: Tubulin alpha-1B chain
| Macromolecule | Name: Tubulin alpha-1B chain / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 49.095438 KDa |
| Sequence | String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPSDK TIGGGDDSFN TFFSETGAGK HVPRAVFVDL EPTVIDEVRT GTYRQLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLVL DRIRKLADQC TGLQGFLVFH SFGGGTGSGF TSLLMERLSV D YGKKSKLE ...String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPSDK TIGGGDDSFN TFFSETGAGK HVPRAVFVDL EPTVIDEVRT GTYRQLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLVL DRIRKLADQC TGLQGFLVFH SFGGGTGSGF TSLLMERLSV D YGKKSKLE FSIYPAPQVS TAVVEPYNSI LTTHTTLEHS DCAFMVDNEA IYDICRRNLD IERPTYTNLN RLISQIVSSI TA SLRFDGA LNVDLTEFQT NLVPYPRIHF PLATYAPVIS AEKAYHEQLS VAEITNACFE PANQMVKCDP RHGKYMACCL LYR GDVVPK DVNAAIATIK TKRSIQFVDW CPTGFKVGIN YQPPTVVPGG DLAKVQRAVC MLSNTTAIAE AWARLDHKFD LMYA KRAFV HWYVGEGMEE GEFSEAREDM AALEKDYEEV GVDSVE UniProtKB: Tubulin alpha-1B chain |
-Macromolecule #2: Tubulin beta chain
| Macromolecule | Name: Tubulin beta chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 48.299293 KDa |
| Sequence | String: MREIVHIQAG QCGNQIGAKF WEVISDEHGI DPTGSYHGDS DLQLERINVY YNEAAGNKYV PRAILVDLEP GTMDSVRSGP FGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV VRKESESCDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS ...String: MREIVHIQAG QCGNQIGAKF WEVISDEHGI DPTGSYHGDS DLQLERINVY YNEAAGNKYV PRAILVDLEP GTMDSVRSGP FGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV VRKESESCDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS VVPSPKVSDT VVEPYNATLS VHQLVENTDE TYCIDNEALY DICFRTLKLT TPTYGDLNHL VSATMSGVTT CL RFPGQLN ADLRKLAVNM VPFPRLHFFM PGFAPLTSRG SQQYRALTVP ELTQQMFDAK NMMAACDPRH GRYLTVAAVF RGR MSMKEV DEQMLNVQNK NSSYFVEWIP NNVKTAVCDI PPRGLKMSAT FIGNSTAIQE LFKRISEQFT AMFRRKAFLH WYTG EGMDE MEFTEAESNM NDLVSEYQQY QDATAD UniProtKB: Tubulin beta chain |
-Macromolecule #3: Protein regulator of cytokinesis 1
| Macromolecule | Name: Protein regulator of cytokinesis 1 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.69394 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GAAALKNYYE VHKELFEGVQ KWEETWRLFL EFERKASDPN RFTNRGGNLL KEEKQRAKLQ KMLPKLEEEL KARIELWEQE HSKAFMVNG QKFMEYVAEQ WEMHRLEKER AKQERQLKNK KQTETEMLY UniProtKB: Protein regulator of cytokinesis 1 |
-Macromolecule #4: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 1 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #6: GUANOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-DIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 1 / Formula: GDP |
|---|---|
| Molecular weight | Theoretical: 443.201 Da |
| Chemical component information |  ChemComp-GDP: |
-Macromolecule #7: Peloruside A
| Macromolecule | Name: Peloruside A / type: ligand / ID: 7 / Number of copies: 1 / Formula: POU |
|---|---|
| Molecular weight | Theoretical: 548.663 Da |
| Chemical component information | 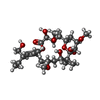 ChemComp-POU: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 6.8 Component:
| ||||||||||||||||||
| Grid | Model: protochips CF-1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 20 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: blot for 4 seconds, blot force 10. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Temperature | Min: 93.0 K / Max: 93.0 K |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3710 pixel / Digitization - Dimensions - Height: 3838 pixel / Digitization - Frames/image: 1-20 / Number grids imaged: 1 / Number real images: 312 / Average exposure time: 0.3 sec. / Average electron dose: 8.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 3.8000000000000003 µm / Calibrated defocus min: 1.1 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 27500 |
| Sample stage | Specimen holder model: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 8.67 Å Applied symmetry - Helical parameters - Δ&Phi: -25.76 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Algorithm: FOURIER SPACE / Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: FREALIGN (ver. 9.09) / Number images used: 17069 |
|---|---|
| Segment selection | Number selected: 27275 Details: manually selected microtubule filaments using manualpicker.py |
| Startup model | Type of model: OTHER Details: low-pass filtered reference model of microtubules decorated with kinesin |
| Final angle assignment | Type: NOT APPLICABLE / Software - Name: FREALIGN (ver. 9.09) |
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: correlation coefficient | ||||||
| Output model |  PDB-5kmg: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)























