[English] 日本語
 Yorodumi
Yorodumi- EMDB-7771: Cryo-EM reconstruction of microtubule-bound synthetic (R2x4) tau -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7771 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of microtubule-bound synthetic (R2x4) tau | |||||||||
 Map data Map data | Cryo-EM reconstruction of microtubule-bound synthetic tau (R2x4) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | microtubule / tau / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationMicrotubule-dependent trafficking of connexons from Golgi to the plasma membrane / Resolution of Sister Chromatid Cohesion / Hedgehog 'off' state / Cilium Assembly / Intraflagellar transport / COPI-dependent Golgi-to-ER retrograde traffic / Mitotic Prometaphase / Carboxyterminal post-translational modifications of tubulin / RHOH GTPase cycle / EML4 and NUDC in mitotic spindle formation ...Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Resolution of Sister Chromatid Cohesion / Hedgehog 'off' state / Cilium Assembly / Intraflagellar transport / COPI-dependent Golgi-to-ER retrograde traffic / Mitotic Prometaphase / Carboxyterminal post-translational modifications of tubulin / RHOH GTPase cycle / EML4 and NUDC in mitotic spindle formation / Sealing of the nuclear envelope (NE) by ESCRT-III / Kinesins / PKR-mediated signaling / Separation of Sister Chromatids / The role of GTSE1 in G2/M progression after G2 checkpoint / Aggrephagy / RHO GTPases activate IQGAPs / RHO GTPases Activate Formins / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / MHC class II antigen presentation / Recruitment of NuMA to mitotic centrosomes / plus-end-directed organelle transport along microtubule / histone-dependent DNA binding / COPI-mediated anterograde transport / negative regulation of protein localization to mitochondrion / neurofibrillary tangle / microtubule lateral binding / axonal transport / tubulin complex / positive regulation of protein localization to synapse / phosphatidylinositol bisphosphate binding / generation of neurons / rRNA metabolic process / axonal transport of mitochondrion / regulation of mitochondrial fission / axon development / regulation of microtubule-based movement / regulation of chromosome organization / central nervous system neuron development / intracellular distribution of mitochondria / minor groove of adenine-thymine-rich DNA binding / lipoprotein particle binding / microtubule polymerization / negative regulation of mitochondrial membrane potential / regulation of microtubule polymerization / dynactin binding / apolipoprotein binding / main axon / protein polymerization / axolemma / Caspase-mediated cleavage of cytoskeletal proteins / regulation of microtubule polymerization or depolymerization / negative regulation of mitochondrial fission / glial cell projection / neurofibrillary tangle assembly / positive regulation of axon extension / regulation of cellular response to heat / Activation of AMPK downstream of NMDARs / positive regulation of superoxide anion generation / positive regulation of protein localization / regulation of long-term synaptic depression / cellular response to brain-derived neurotrophic factor stimulus / positive regulation of microtubule polymerization / supramolecular fiber organization / synapse assembly / cytoplasmic microtubule organization / regulation of calcium-mediated signaling / somatodendritic compartment / axon cytoplasm / phosphatidylinositol binding / astrocyte activation / enzyme inhibitor activity / nuclear periphery / stress granule assembly / protein phosphatase 2A binding / regulation of microtubule cytoskeleton organization / cellular response to reactive oxygen species / Hsp90 protein binding / microglial cell activation / cellular response to nerve growth factor stimulus / synapse organization / PKR-mediated signaling / regulation of synaptic plasticity / protein homooligomerization / regulation of autophagy / response to lead ion / structural constituent of cytoskeleton / SH3 domain binding / microtubule cytoskeleton organization / memory / neuron migration / cytoplasmic ribonucleoprotein granule / neuron projection development / cell-cell signaling / mitotic cell cycle / single-stranded DNA binding / protein-folding chaperone binding / cellular response to heat / microtubule cytoskeleton / actin binding Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Nogales E / Kellogg EH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2018 Journal: Science / Year: 2018Title: Near-atomic model of microtubule-tau interactions. Authors: Elizabeth H Kellogg / Nisreen M A Hejab / Simon Poepsel / Kenneth H Downing / Frank DiMaio / Eva Nogales /  Abstract: Tau is a developmentally regulated axonal protein that stabilizes and bundles microtubules (MTs). Its hyperphosphorylation is thought to cause detachment from MTs and subsequent aggregation into ...Tau is a developmentally regulated axonal protein that stabilizes and bundles microtubules (MTs). Its hyperphosphorylation is thought to cause detachment from MTs and subsequent aggregation into fibrils implicated in Alzheimer's disease. It is unclear which tau residues are crucial for tau-MT interactions, where tau binds on MTs, and how it stabilizes them. We used cryo-electron microscopy to visualize different tau constructs on MTs and computational approaches to generate atomic models of tau-tubulin interactions. The conserved tubulin-binding repeats within tau adopt similar extended structures along the crest of the protofilament, stabilizing the interface between tubulin dimers. Our structures explain the effect of phosphorylation on MT affinity and lead to a model of tau repeats binding in tandem along protofilaments, tethering together tubulin dimers and stabilizing polymerization interfaces. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7771.map.gz emd_7771.map.gz | 278.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7771-v30.xml emd-7771-v30.xml emd-7771.xml emd-7771.xml | 19.7 KB 19.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_7771.png emd_7771.png | 152.3 KB | ||
| Filedesc metadata |  emd-7771.cif.gz emd-7771.cif.gz | 7.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7771 http://ftp.pdbj.org/pub/emdb/structures/EMD-7771 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7771 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7771 | HTTPS FTP |
-Related structure data
| Related structure data |  6cvnMC  7520C  7522C  7523C  7769C  6cvjC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7771.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7771.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM reconstruction of microtubule-bound synthetic tau (R2x4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Ternary complex of alpha-beta tubulin with synthetic (R2x4) tau
+Supramolecule #1: Ternary complex of alpha-beta tubulin with synthetic (R2x4) tau
+Supramolecule #2: Tubulin beta chain
+Supramolecule #3: Tubulin alpha-1B chain
+Supramolecule #4: Microtubule-associated protein tau
+Macromolecule #1: Tubulin beta chain
+Macromolecule #2: Tubulin alpha-1B chain
+Macromolecule #3: Microtubule-associated protein tau
+Macromolecule #4: GUANOSINE-5'-DIPHOSPHATE
+Macromolecule #5: GUANOSINE-5'-TRIPHOSPHATE
+Macromolecule #6: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 6.8 Component:
| ||||||||||||||||||
| Grid | Model: C-flat-1.2/1.3 4C / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 10 sec. / Pretreatment - Atmosphere: AIR | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 310.15 K / Instrument: FEI VITROBOT MARK IV Details: blot force 10 pN, 6 second blot time. used C-flat 1.2/1.3 holey grids. First 2 uL of microtubules were adhered to grid for 30 seconds, followed by 2 4 uL washes of tau with 30 second incubation for each wash.. | ||||||||||||||||||
| Details | tubulin concentration is 0.5 mg/mL, tau concentration is 1 mg/mL |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE |
| Image recording | Film or detector model: GATAN K2 BASE (4k x 4k) / Number grids imaged: 1 / Number real images: 812 / Average exposure time: 10.0 sec. / Average electron dose: 40.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus min: 0.5 µm / Nominal magnification: 37878 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 8.7 Å Applied symmetry - Helical parameters - Δ&Phi: -25.75 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Algorithm: FOURIER SPACE / Resolution.type: BY AUTHOR / Resolution: 3.9 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: FREALIGN (ver. 9.09) / Number images used: 19505 |
|---|---|
| Segment selection | Number selected: 24571 / Software - Name: Appion |
| Startup model | Type of model: EMDB MAP EMDB ID: Details: used naked microtubule reconstruction as initial model, low-pass filtered to 20 Angstrom |
| Final angle assignment | Type: NOT APPLICABLE / Software - Name: FREALIGN (ver. 9.09) |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 120 / Target criteria: real space correlation |
|---|---|
| Output model |  PDB-6cvn: |
 Movie
Movie Controller
Controller


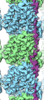











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)