[English] 日本語
 Yorodumi
Yorodumi- EMDB-6121: Cryo-EM reconstruction of quasi-HPV16 complexed with H16.14J Fab -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6121 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of quasi-HPV16 complexed with H16.14J Fab | |||||||||
 Map data Map data | Reconstruction of quasi-HPV16 complexed with H16.14J Fab | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | quasi-HPV16 / L1 capsomer / H16.14J Fab | |||||||||
| Function / homology |  Function and homology information Function and homology informationT=7 icosahedral viral capsid / endocytosis involved in viral entry into host cell / virion attachment to host cell / host cell nucleus / structural molecule activity Similarity search - Function | |||||||||
| Biological species |   Human papillomavirus 16 Human papillomavirus 16 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 13.9 Å | |||||||||
 Authors Authors | Guan J / Bywaters SM / Brendle SA / Lee H / Ashley RE / Conway JF / Makhov AM / Christensen ND / Hafenstein S | |||||||||
 Citation Citation |  Journal: Virology / Year: 2015 Journal: Virology / Year: 2015Title: Structural comparison of four different antibodies interacting with human papillomavirus 16 and mechanisms of neutralization. Authors: Jian Guan / Stephanie M Bywaters / Sarah A Brendle / Hyunwook Lee / Robert E Ashley / Alexander M Makhov / James F Conway / Neil D Christensen / Susan Hafenstein /  Abstract: Cryo-electron microscopy (cryo-EM) was used to solve the structures of human papillomavirus type 16 (HPV16) complexed with fragments of antibody (Fab) from three different neutralizing monoclonals ...Cryo-electron microscopy (cryo-EM) was used to solve the structures of human papillomavirus type 16 (HPV16) complexed with fragments of antibody (Fab) from three different neutralizing monoclonals (mAbs): H16.1A, H16.14J, and H263.A2. The structure-function analysis revealed predominantly monovalent binding of each Fab with capsid interactions that involved multiple loops from symmetry related copies of the major capsid protein. The residues identified in each Fab-virus interface map to a conformational groove on the surface of the capsomer. In addition to the known involvement of the FG and HI loops, the DE loop was also found to constitute the core of each epitope. Surprisingly, the epitope mapping also identified minor contributions by EF and BC loops. Complementary immunological assays included mAb and Fab neutralization. The specific binding characteristics of mAbs correlated with different neutralizing behaviors in pre- and post-attachment neutralization assays. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6121.map.gz emd_6121.map.gz | 127 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6121-v30.xml emd-6121-v30.xml emd-6121.xml emd-6121.xml | 12.4 KB 12.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_6121.tif emd_6121.tif | 1000.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6121 http://ftp.pdbj.org/pub/emdb/structures/EMD-6121 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6121 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6121 | HTTPS FTP |
-Related structure data
| Related structure data |  3j8vMC  5990C  6184C  3j8wC  3j8zC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6121.map.gz / Format: CCP4 / Size: 379.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6121.map.gz / Format: CCP4 / Size: 379.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of quasi-HPV16 complexed with H16.14J Fab | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.33 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Quasi-human papillomavirus 16 complexed with H16.14J Fab
| Entire | Name: Quasi-human papillomavirus 16 complexed with H16.14J Fab |
|---|---|
| Components |
|
-Supramolecule #1000: Quasi-human papillomavirus 16 complexed with H16.14J Fab
| Supramolecule | Name: Quasi-human papillomavirus 16 complexed with H16.14J Fab type: sample / ID: 1000 / Oligomeric state: 300 H16.14J Fabs bind to one HPV16 capsid / Number unique components: 2 |
|---|---|
| Molecular weight | Theoretical: 44.7 MDa |
-Supramolecule #1: Human papillomavirus 16
| Supramolecule | Name: Human papillomavirus 16 / type: virus / ID: 1 / NCBI-ID: 337041 / Sci species name: Human papillomavirus 16 / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) / synonym: VERTEBRATES Homo sapiens (human) / synonym: VERTEBRATES |
| Host system | Organism:  Homo sapiens (human) / Recombinant cell: 293TT / Recombinant plasmid: SV40 Homo sapiens (human) / Recombinant cell: 293TT / Recombinant plasmid: SV40 |
| Molecular weight | Theoretical: 27.7 MDa |
| Virus shell | Shell ID: 1 / Name: L1 L2 / Diameter: 600 Å / T number (triangulation number): 7 |
-Macromolecule #1: H16.14J Fab
| Macromolecule | Name: H16.14J Fab / type: protein_or_peptide / ID: 1 / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 1 M NaCl, 200 mM Tris |
| Grid | Details: glow-discharged holey carbon support grid |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 102 K / Instrument: GATAN CRYOPLUNGE 3 / Method: Blot for 0.7 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 2100 |
|---|---|
| Temperature | Average: 95 K |
| Date | Aug 27, 2014 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Number real images: 385 / Average electron dose: 15 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 4.69 µm / Nominal defocus min: 0.62 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Details | The particles were selected using semi-automatic program e2boxer.py (EMAN2). |
|---|---|
| CTF correction | Details: Each particle |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 13.9 Å / Resolution method: OTHER / Software - Name: Auto3dem Details: Semi-automatic particle selection was performed using e2boxer.py to obtain the particle coordinates, followed by particle boxing, linearization, normalization, and apodization of the images ...Details: Semi-automatic particle selection was performed using e2boxer.py to obtain the particle coordinates, followed by particle boxing, linearization, normalization, and apodization of the images using Robem. Defocus and astigmatism values necessary to perform contrast transfer function (CTF) correction for the extracted particles were assessed using Robem. The icosahedrally averaged reconstruction was initiated using a random model generated with setup_rmc. For the last step of refinement, the final maps were CTF-corrected using a B factor of 200 A2. Number images used: 5642 |
-Atomic model buiding 1
| Initial model | PDB ID:  3oae Chain - #0 - Chain ID: A / Chain - #1 - Chain ID: B / Chain - #2 - Chain ID: C / Chain - #3 - Chain ID: D / Chain - #4 - Chain ID: E |
|---|---|
| Software | Name: Chimera, Situs |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-3j8v: |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera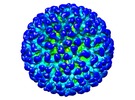












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)