+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Mycobacterium smegmatis inosine monophosphate dehydrogenase (IMPDH) E-XMP* intermediate, compressed | |||||||||
 マップデータ マップデータ | LocScale primary viewing map | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Octamer / reaction intermediate / Purine metabolism / IMPDH / OXIDOREDUCTASE | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報IMP dehydrogenase / IMP dehydrogenase activity / GMP biosynthetic process / GTP biosynthetic process / nucleotide binding / metal ion binding 類似検索 - 分子機能 | |||||||||
| 生物種 |  Mycolicibacterium smegmatis MC2 155 (バクテリア) Mycolicibacterium smegmatis MC2 155 (バクテリア) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 2.73 Å | |||||||||
 データ登録者 データ登録者 | Bulvas O / Kouba T / Pichova I | |||||||||
| 資金援助 | European Union, 1件
| |||||||||
 引用 引用 |  ジャーナル: J Struct Biol / 年: 2025 ジャーナル: J Struct Biol / 年: 2025タイトル: Conformational landscape of the mycobacterial inosine 5'-monophosphate dehydrogenase octamerization interface. 著者: Ondřej Bulvas / Zdeněk Knejzlík / Anatolij Filimoněnko / Tomáš Kouba / Iva Pichová /  要旨: Inosine 5'-monophosphate dehydrogenase (IMPDH), a key enzyme in bacterial purine metabolism, plays an essential role in the biosynthesis of guanine nucleotides and shows promise as a target for ...Inosine 5'-monophosphate dehydrogenase (IMPDH), a key enzyme in bacterial purine metabolism, plays an essential role in the biosynthesis of guanine nucleotides and shows promise as a target for antimicrobial drug development. Despite its significance, the conformational dynamics and substrate-induced structural changes in bacterial IMPDH remain poorly understood, particularly with respect to its octameric assembly. Using cryo-EM, we present full-length structures of IMPDH from Mycobacterium smegmatis (MsmIMPDH) captured in a reaction intermediate state, revealing conformational changes upon substrate binding. The structures feature resolved flexible loops that coordinate the binding of the substrate, the cofactor, and the K ion. Our structural analysis identifies a novel octamerization interface unique to MsmIMPDH. Additionally, a previously unobserved barrel-like density suggests potential self-interactions within the C-terminal regions, hinting at a regulatory mechanism tied to assembly and function of the enzyme. These data provide insights into substrate-induced conformational dynamics and novel interaction interfaces in MsmIMPDH, potentially informing the development of IMPDH-targeted drugs. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_52559.map.gz emd_52559.map.gz | 136 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-52559-v30.xml emd-52559-v30.xml emd-52559.xml emd-52559.xml | 22.8 KB 22.8 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_52559_fsc.xml emd_52559_fsc.xml | 14.8 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_52559.png emd_52559.png | 131.4 KB | ||
| マスクデータ |  emd_52559_msk_1.map emd_52559_msk_1.map | 282.6 MB |  マスクマップ マスクマップ | |
| Filedesc metadata |  emd-52559.cif.gz emd-52559.cif.gz | 7.1 KB | ||
| その他 |  emd_52559_additional_1.map.gz emd_52559_additional_1.map.gz emd_52559_half_map_1.map.gz emd_52559_half_map_1.map.gz emd_52559_half_map_2.map.gz emd_52559_half_map_2.map.gz | 263.1 MB 225.3 MB 225.3 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-52559 http://ftp.pdbj.org/pub/emdb/structures/EMD-52559 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-52559 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-52559 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_52559.map.gz / 形式: CCP4 / 大きさ: 282.6 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_52559.map.gz / 形式: CCP4 / 大きさ: 282.6 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | LocScale primary viewing map | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.8336 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-マスク #1
| ファイル |  emd_52559_msk_1.map emd_52559_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-追加マップ: Original map used for refinement and validation
| ファイル | emd_52559_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Original map used for refinement and validation | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: #2
| ファイル | emd_52559_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_52559_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Octameric assembly of inosine monophosphate dehydrogenase reactio...
| 全体 | 名称: Octameric assembly of inosine monophosphate dehydrogenase reaction intermediate; E-XMP* in complex with NAD+ |
|---|---|
| 要素 |
|
-超分子 #1: Octameric assembly of inosine monophosphate dehydrogenase reactio...
| 超分子 | 名称: Octameric assembly of inosine monophosphate dehydrogenase reaction intermediate; E-XMP* in complex with NAD+ タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1 |
|---|---|
| 由来(天然) | 生物種:  Mycolicibacterium smegmatis MC2 155 (バクテリア) Mycolicibacterium smegmatis MC2 155 (バクテリア) |
-分子 #1: Inosine-5'-monophosphate dehydrogenase
| 分子 | 名称: Inosine-5'-monophosphate dehydrogenase / タイプ: protein_or_peptide / ID: 1 / コピー数: 8 / 光学異性体: LEVO / EC番号: IMP dehydrogenase |
|---|---|
| 由来(天然) | 生物種:  Mycolicibacterium smegmatis MC2 155 (バクテリア) Mycolicibacterium smegmatis MC2 155 (バクテリア)株: MC2 155 |
| 分子量 | 理論値: 53.388988 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MSIAESSVPI AVPVPTGGDD PTKVAMLGLT FDDVLLLPAA SDVVPATADT SSQLTKRIRL RVPLVSSAMD TVTESRMAIA MARAGGMGV LHRNLPVAEQ AGQVETVKRS EAGMVTDPVT CSPDNTLAEV DAMCARFRIS GLPVVDDTGE LVGIITNRDM R FEVDQSKP ...文字列: MSIAESSVPI AVPVPTGGDD PTKVAMLGLT FDDVLLLPAA SDVVPATADT SSQLTKRIRL RVPLVSSAMD TVTESRMAIA MARAGGMGV LHRNLPVAEQ AGQVETVKRS EAGMVTDPVT CSPDNTLAEV DAMCARFRIS GLPVVDDTGE LVGIITNRDM R FEVDQSKP VSEVMTKAPL ITAKEGVSAE AALGLLRRHK IEKLPIVDGH GKLTGLITVK DFVKTEQFPL STKDSDGRLL VG AAVGVGD DAWTRAMTLV DAGVDVLIVD TAHAHNRGVL DMVSRLKQAV GERVDVVGGN VATRAAAAAL VEAGADAVKV GVG PGSICT TRVVAGVGAP QITAILEAVA ACKPYGVPVI ADGGLQYSGD IAKALAAGAS TAMLGSLLAG TAESPGELIF VNGK QFKSY RGMGSLGAMQ GRGAAKSYSK DRYFQDDVLS EDKLVPEGIE GRVPFRGPLG TVIHQLTGGL RAAMGYTGSA TIEQL QQAQ FVQITAAGLK ESHPHDITMT VEAPNYYTR UniProtKB: Inosine-5'-monophosphate dehydrogenase |
-分子 #2: INOSINIC ACID
| 分子 | 名称: INOSINIC ACID / タイプ: ligand / ID: 2 / コピー数: 8 / 式: IMP |
|---|---|
| 分子量 | 理論値: 348.206 Da |
| Chemical component information | 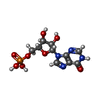 ChemComp-I: |
-分子 #3: NICOTINAMIDE-ADENINE-DINUCLEOTIDE
| 分子 | 名称: NICOTINAMIDE-ADENINE-DINUCLEOTIDE / タイプ: ligand / ID: 3 / コピー数: 8 / 式: NAD |
|---|---|
| 分子量 | 理論値: 663.425 Da |
| Chemical component information |  ChemComp-NAD: |
-分子 #4: POTASSIUM ION
| 分子 | 名称: POTASSIUM ION / タイプ: ligand / ID: 4 / コピー数: 8 / 式: K |
|---|---|
| 分子量 | 理論値: 39.098 Da |
-分子 #5: water
| 分子 | 名称: water / タイプ: ligand / ID: 5 / コピー数: 80 / 式: HOH |
|---|---|
| 分子量 | 理論値: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 構成要素:
詳細: 50 mM HEPES (pH 7.5), 200 mM KCl, 5 mM DTT, 32 mM MgCl2 Ligand: 10 mM ATP + 10 mM IMP + 10 mM NAD | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| グリッド | モデル: Quantifoil R2/1 / 材質: GOLD / メッシュ: 300 / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: HOLEY ARRAY / 前処理 - タイプ: GLOW DISCHARGE | ||||||||||||||||||||||||
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / 装置: FEI VITROBOT MARK IV |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | TFS KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 実像数: 8691 / 平均露光時間: 2.0 sec. / 平均電子線量: 40.1 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 3.2 µm / 最小 デフォーカス(公称値): 1.2 µm / 倍率(公称値): 165000 |
| 試料ステージ | ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 初期モデル | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| 詳細 | Initial fitting was done in UCSF ChimeraX. Model refinement was done by iterative cycles of manual fitting with Coot and ISOLDE and automated fitting with phenix.real_space_refine. |
| 精密化 | プロトコル: RIGID BODY FIT / 温度因子: 37.48 / 当てはまり具合の基準: CC coefficient |
| 得られたモデル |  PDB-9i0k: |
 ムービー
ムービー コントローラー
コントローラー









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)























































