[English] 日本語
 Yorodumi
Yorodumi- EMDB-4542: CryoEM structure of a beta3K279T GABA(A)R homomer in complex with... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4542 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of a beta3K279T GABA(A)R homomer in complex with histamine and megabody Mb25 | ||||||||||||||||||
 Map data Map data | Final EM map from RELION 3.0 Postprocess job | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Megabody / Membrane protein / protein engineering / cryo-EM | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcircadian sleep/wake cycle, REM sleep / reproductive behavior / hard palate development / cellular response to histamine / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-A receptor activity / GABA-gated chloride ion channel activity / GABA-A receptor complex ...circadian sleep/wake cycle, REM sleep / reproductive behavior / hard palate development / cellular response to histamine / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-A receptor activity / GABA-gated chloride ion channel activity / GABA-A receptor complex / innervation / response to anesthetic / postsynaptic specialization membrane / inhibitory postsynaptic potential / gamma-aminobutyric acid signaling pathway / synaptic transmission, GABAergic / cellular response to zinc ion / exploration behavior / motor behavior / roof of mouth development / Signaling by ERBB4 / cochlea development / social behavior / chloride channel complex / chloride transmembrane transport / cytoplasmic vesicle membrane / cerebellum development / learning / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / GABA-ergic synapse / memory / dendritic spine / postsynaptic membrane / response to xenobiotic stimulus / cell surface / signal transduction / identical protein binding / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.49 Å | ||||||||||||||||||
 Authors Authors | Uchanski T / Masiulis S | ||||||||||||||||||
| Funding support |  United Kingdom, United Kingdom,  Belgium, 5 items Belgium, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Methods / Year: 2021 Journal: Nat Methods / Year: 2021Title: Megabodies expand the nanobody toolkit for protein structure determination by single-particle cryo-EM. Authors: Tomasz Uchański / Simonas Masiulis / Baptiste Fischer / Valentina Kalichuk / Uriel López-Sánchez / Eleftherios Zarkadas / Miriam Weckener / Andrija Sente / Philip Ward / Alexandre ...Authors: Tomasz Uchański / Simonas Masiulis / Baptiste Fischer / Valentina Kalichuk / Uriel López-Sánchez / Eleftherios Zarkadas / Miriam Weckener / Andrija Sente / Philip Ward / Alexandre Wohlkönig / Thomas Zögg / Han Remaut / James H Naismith / Hugues Nury / Wim Vranken / A Radu Aricescu / Els Pardon / Jan Steyaert /    Abstract: Nanobodies are popular and versatile tools for structural biology. They have a compact single immunoglobulin domain organization, bind target proteins with high affinities while reducing their ...Nanobodies are popular and versatile tools for structural biology. They have a compact single immunoglobulin domain organization, bind target proteins with high affinities while reducing their conformational heterogeneity and stabilize multi-protein complexes. Here we demonstrate that engineered nanobodies can also help overcome two major obstacles that limit the resolution of single-particle cryo-electron microscopy reconstructions: particle size and preferential orientation at the water-air interfaces. We have developed and characterized constructs, termed megabodies, by grafting nanobodies onto selected protein scaffolds to increase their molecular weight while retaining the full antigen-binding specificity and affinity. We show that the megabody design principles are applicable to different scaffold proteins and recognition domains of compatible geometries and are amenable for efficient selection from yeast display libraries. Moreover, we demonstrate that megabodies can be used to obtain three-dimensional reconstructions for membrane proteins that suffer from severe preferential orientation or are otherwise too small to allow accurate particle alignment. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4542.map.gz emd_4542.map.gz | 6.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4542-v30.xml emd-4542-v30.xml emd-4542.xml emd-4542.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4542.png emd_4542.png | 163.4 KB | ||
| Masks |  emd_4542_msk_1.map emd_4542_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-4542.cif.gz emd-4542.cif.gz | 7.2 KB | ||
| Others |  emd_4542_additional_1.map.gz emd_4542_additional_1.map.gz emd_4542_half_map_1.map.gz emd_4542_half_map_1.map.gz emd_4542_half_map_2.map.gz emd_4542_half_map_2.map.gz | 49.3 MB 49.6 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4542 http://ftp.pdbj.org/pub/emdb/structures/EMD-4542 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4542 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4542 | HTTPS FTP |
-Related structure data
| Related structure data |  6qfaMC  6xuxC  6xv8C  6xviC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4542.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4542.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final EM map from RELION 3.0 Postprocess job | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_4542_msk_1.map emd_4542_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Combined, unfiltered/unsharpened map from Relion Refine3D
| File | emd_4542_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Combined, unfiltered/unsharpened map from Relion Refine3D | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map from Relion
| File | emd_4542_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map from Relion | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map from Relion
| File | emd_4542_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map from Relion | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Homomeric truncated (M3-M4 loop) GABAAR b3K279T receptor in compl...
| Entire | Name: Homomeric truncated (M3-M4 loop) GABAAR b3K279T receptor in complex with histamine and megabody Mb25 |
|---|---|
| Components |
|
-Supramolecule #1: Homomeric truncated (M3-M4 loop) GABAAR b3K279T receptor in compl...
| Supramolecule | Name: Homomeric truncated (M3-M4 loop) GABAAR b3K279T receptor in complex with histamine and megabody Mb25 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Molecular weight | Theoretical: 500 KDa |
-Supramolecule #2: Homomeric truncated (M3-M4 loop) GABAAR b3K279T receptor
| Supramolecule | Name: Homomeric truncated (M3-M4 loop) GABAAR b3K279T receptor type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: Brain / Location in cell: Plasma membrane Homo sapiens (human) / Organ: Brain / Location in cell: Plasma membrane |
-Supramolecule #3: Megabody Mb25
| Supramolecule | Name: Megabody Mb25 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Gamma-aminobutyric acid receptor subunit beta-3,Gamma-aminobutyri...
| Macromolecule | Name: Gamma-aminobutyric acid receptor subunit beta-3,Gamma-aminobutyric acid receptor subunit beta-3 type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.503555 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QSVNDPGNMS FVKETVDKLL KGYDIRLRPD FGGPPVCVGM NIDIASIDMV SEVNMDYTLT MYFQQYWRDK RLAYSGIPLN LTLDNRVAD QLWVPDTYFL NDKKSFVHGV TVKNRMIRLH PDGTVLYGLR ITTTAACMMD LRRYPLDEQN CTLEIESYGY T TDDIEFYW ...String: QSVNDPGNMS FVKETVDKLL KGYDIRLRPD FGGPPVCVGM NIDIASIDMV SEVNMDYTLT MYFQQYWRDK RLAYSGIPLN LTLDNRVAD QLWVPDTYFL NDKKSFVHGV TVKNRMIRLH PDGTVLYGLR ITTTAACMMD LRRYPLDEQN CTLEIESYGY T TDDIEFYW RGGDKAVTGV ERIELPQFSI VEHRLVSRNV VFATGAYPRL SLSFRLKRNI GYFILQTYMP SILITILSWV SF WINYDAS AARVALGITT VLTMTTINTH LRETLPKIPY VTAIDMYLMG CFVFVFLALL EYAFVNYIFF SQPARAAAID RWS RIVFPF TFSLFNLVYW LYYVN UniProtKB: Gamma-aminobutyric acid receptor subunit beta-3, Gamma-aminobutyric acid receptor subunit beta-3 |
-Macromolecule #2: Outer membrane protein,Outer membrane protein,Outer membrane prot...
| Macromolecule | Name: Outer membrane protein,Outer membrane protein,Outer membrane protein,Outer membrane protein,Uncharacterized protein,Uncharacterized protein,Mb-c7HopQ-Nb25 type: protein_or_peptide / ID: 2 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 56.300629 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLVESGGG LVQTKTTTSV IDTTNDAQNL LTQAQTIVNT LKDYCPILIA KSSSSNGGTN NANTPSWQTA GGGKNSCATF GAEFSAASD MINNAQKIVQ ETQQLSANQP KNITQPHNLN LNSPSSLTAL AQKMLKNAQS QAEILKLANQ VESDFNKLSS G HLKDYIGK ...String: QVQLVESGGG LVQTKTTTSV IDTTNDAQNL LTQAQTIVNT LKDYCPILIA KSSSSNGGTN NANTPSWQTA GGGKNSCATF GAEFSAASD MINNAQKIVQ ETQQLSANQP KNITQPHNLN LNSPSSLTAL AQKMLKNAQS QAEILKLANQ VESDFNKLSS G HLKDYIGK CDASAISSAN MTMQNQKNNW GNGCAGVEET QSLLKTSAAD FNNQTPQINQ AQNLANTLIQ ELGNNTYEQL SR LLTNDNG TNSKTSAQAI NQAVNNLNER AKTLAGGTTN SPAYQATLLA LRSVLGLWNS MGYAVICGGY TKSPGENNQK DFH YTDENG NGTTINCGGS TNSNGTHSYN GTNTLKADKN VSLSIEQYEK IHEAYQILSK ALKQAGLAPL NSKGEKLEAH VTTS KYGSL RLSCAASGHT FNYPIMGWFR QAPGKEREFV GAISWSGGST SYADSVKDRF TISRDNAKNT VYLEMNNLKP EDTAV YYCA AKGRYSGGLY YPTNYDYWGQ GTQVTVSSHH HHHHEPEA UniProtKB: Outer membrane protein, Outer membrane protein |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 5 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #5: HISTAMINE
| Macromolecule | Name: HISTAMINE / type: ligand / ID: 5 / Number of copies: 5 / Formula: HSM |
|---|---|
| Molecular weight | Theoretical: 111.145 Da |
| Chemical component information | 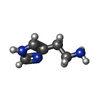 ChemComp-HSM: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL |
|---|---|
| Buffer | pH: 7.6 |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
| Details | Monodisperse sample |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 0.7000000000000001 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-6qfa: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















































