+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human ATG9A in LMNG micelles | |||||||||
 Map data Map data | The autophagy relate transmembrane protein ATG9A, lipid scramblase | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Lipid scramblase / ATG9A / single particle cryo-EM / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationphospholipid scramblase activity / programmed necrotic cell death / protein localization to phagophore assembly site / phagophore assembly site membrane / piecemeal microautophagy of the nucleus / phagophore assembly site / bone morphogenesis / reticulophagy / autophagy of mitochondrion / Macroautophagy ...phospholipid scramblase activity / programmed necrotic cell death / protein localization to phagophore assembly site / phagophore assembly site membrane / piecemeal microautophagy of the nucleus / phagophore assembly site / bone morphogenesis / reticulophagy / autophagy of mitochondrion / Macroautophagy / autophagosome assembly / autophagosome / PINK1-PRKN Mediated Mitophagy / mitochondrial membrane / trans-Golgi network / recycling endosome / recycling endosome membrane / late endosome / late endosome membrane / endosome / Golgi membrane / intracellular membrane-bounded organelle / endoplasmic reticulum membrane / Golgi apparatus / mitochondrion / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
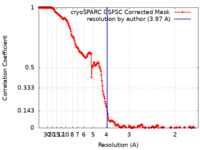
| Method | single particle reconstruction / cryo EM / Resolution: 3.97 Å | |||||||||
 Authors Authors | Yang W / Goran S | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Structural basis for lipid transfer by the ATG2A-ATG9A complex. Authors: Yang Wang / Selma Dahmane / Rujuan Ti / Xinyi Mai / Lizhe Zhu / Lars-Anders Carlson / Goran Stjepanovic /   Abstract: Autophagy is characterized by the formation of double-membrane vesicles called autophagosomes. Autophagy-related proteins (ATGs) 2A and 9A have an essential role in autophagy by mediating lipid ...Autophagy is characterized by the formation of double-membrane vesicles called autophagosomes. Autophagy-related proteins (ATGs) 2A and 9A have an essential role in autophagy by mediating lipid transfer and re-equilibration between membranes for autophagosome formation. Here we report the cryo-electron microscopy structures of human ATG2A in complex with WD-repeat protein interacting with phosphoinositides 4 (WIPI4) at 3.2 Å and the ATG2A-WIPI4-ATG9A complex at 7 Å global resolution. On the basis of molecular dynamics simulations, we propose a mechanism of lipid extraction from the donor membranes. Our analysis revealed 3:1 stoichiometry of the ATG9A-ATG2A complex, directly aligning the ATG9A lateral pore with ATG2A lipid transfer cavity, and an interaction of the ATG9A trimer with both the N-terminal and the C-terminal tip of rod-shaped ATG2A. Cryo-electron tomography of ATG2A liposome-binding states showed that ATG2A tethers lipid vesicles at different orientations. In summary, this study provides a molecular basis for the growth of the phagophore membrane and lends structural insights into spatially coupled lipid transport and re-equilibration during autophagosome formation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37088.map.gz emd_37088.map.gz | 726.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37088-v30.xml emd-37088-v30.xml emd-37088.xml emd-37088.xml | 14.2 KB 14.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_37088_fsc.xml emd_37088_fsc.xml | 20 KB | Display |  FSC data file FSC data file |
| Images |  emd_37088.png emd_37088.png | 68.6 KB | ||
| Filedesc metadata |  emd-37088.cif.gz emd-37088.cif.gz | 5.8 KB | ||
| Others |  emd_37088_half_map_1.map.gz emd_37088_half_map_1.map.gz emd_37088_half_map_2.map.gz emd_37088_half_map_2.map.gz | 764.8 MB 764.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37088 http://ftp.pdbj.org/pub/emdb/structures/EMD-37088 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37088 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37088 | HTTPS FTP |
-Validation report
| Summary document |  emd_37088_validation.pdf.gz emd_37088_validation.pdf.gz | 633.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_37088_full_validation.pdf.gz emd_37088_full_validation.pdf.gz | 633.2 KB | Display | |
| Data in XML |  emd_37088_validation.xml.gz emd_37088_validation.xml.gz | 28.1 KB | Display | |
| Data in CIF |  emd_37088_validation.cif.gz emd_37088_validation.cif.gz | 37.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37088 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37088 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37088 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-37088 | HTTPS FTP |
-Related structure data
| Related structure data |  8kbzMC  8kbxC  8kbyC  8kc3C  8y1lC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37088.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37088.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The autophagy relate transmembrane protein ATG9A, lipid scramblase | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size |
| ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map A of ATG9A
| File | emd_37088_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A of ATG9A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : ATG9A trimer
| Entire | Name: ATG9A trimer |
|---|---|
| Components |
|
-Supramolecule #1: ATG9A trimer
| Supramolecule | Name: ATG9A trimer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 283 KDa |
-Macromolecule #1: Autophagy-related protein 9A
| Macromolecule | Name: Autophagy-related protein 9A / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 94.551031 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAQFDTEYQR LEASYSDSPP GEEDLLVHVA EGSKSPWHHI ENLDLFFSRV YNLHQKNGFT CMLIGEIFEL MQFLFVVAFT TFLVSCVDY DILFANKMVN HSLHPTEPVK VTLPDAFLPA QVCSARIQEN GSLITILVIA GVFWIHRLIK FIYNICCYWE I HSFYLHAL ...String: MAQFDTEYQR LEASYSDSPP GEEDLLVHVA EGSKSPWHHI ENLDLFFSRV YNLHQKNGFT CMLIGEIFEL MQFLFVVAFT TFLVSCVDY DILFANKMVN HSLHPTEPVK VTLPDAFLPA QVCSARIQEN GSLITILVIA GVFWIHRLIK FIYNICCYWE I HSFYLHAL RIPMSALPYC TWQEVQARIV QTQKEHQICI HKRELTELDI YHRILRFQNY MVALVNKSLL PLRFRLPGLG EA VFFTRGL KYNFELILFW GPGSLFLNEW SLKAEYKRGG QRLELAQRLS NRILWIGIAN FLLCPLILIW QILYAFFSYA EVL KREPGA LGARCWSLYG RCYLRHFNEL EHELQSRLNR GYKPASKYMN CFLSPLLTLL AKNGAFFAGS ILAVLIALTI YDED VLAVE HVLTTVTLLG VTVTVCRSFI PDQHMVFCPE QLLRVILAHI HYMPDHWQGN AHRSQTRDEF AQLFQYKAVF ILEEL LSPI VTPLILIFCL RPRALEIIDF FRNFTVEVVG VGDTCSFAQM DVRQHGHPQW LSAGQTEASV YQQAEDGKTE LSLMHF AIT NPGWQPPRES TAFLGFLKEQ VQRDGAAASL AQGGLLPENA LFTSIQSLQS ESEPLSLIAN VVAGSSCRGP PLPRDLQ GS RHRAEVASAL RSFSPLQPGQ APTGRAHSTM TGSGVDARTA SSGSSVWEGQ LQSLVLSEYA STEMSLHALY MHQLHKQQ A QAEPERHVWH RRESDESGES APDEGGEGAR APQSIPRSAS YPCAAPRPGA PETTALHGGF QRRYGGITDP GTVPRVPSH FSRLPLGGWA EDGQSASRHP EPVPEEGSED ELPPQVHKV UniProtKB: Autophagy-related protein 9A |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 65.17 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.9000000000000001 µm / Nominal defocus min: 1.4000000000000001 µm / Nominal magnification: 105000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)