[English] 日本語
 Yorodumi
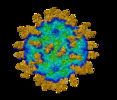
Yorodumi- EMDB-30815: Cryo-EM structure of Coxsackievirus B1 mature virion in complex w... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30815 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Coxsackievirus B1 mature virion in complex with nAb 5F5 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Coxsackievirus B1 / CAR / Cryo-EM / neutralizing antibody / VIRUS / VIRUS-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / viral capsid / nucleoside-triphosphate phosphatase ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / viral capsid / nucleoside-triphosphate phosphatase / host cell / channel activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated suppression of host gene expression / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / symbiont entry into host cell / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |  Coxsackievirus B1 / Coxsackievirus B1 /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Li S / Zhu R | |||||||||
 Citation Citation |  Journal: Cell Host Microbe / Year: 2021 Journal: Cell Host Microbe / Year: 2021Title: Cryo-EM structures reveal the molecular basis of receptor-initiated coxsackievirus uncoating. Authors: Longfa Xu / Qingbing Zheng / Rui Zhu / Zhichao Yin / Hai Yu / Yu Lin / Yuanyuan Wu / Maozhou He / Yang Huang / Yichao Jiang / Hui Sun / Zhenghui Zha / Hongwei Yang / Qiongzi Huang / Dongqing ...Authors: Longfa Xu / Qingbing Zheng / Rui Zhu / Zhichao Yin / Hai Yu / Yu Lin / Yuanyuan Wu / Maozhou He / Yang Huang / Yichao Jiang / Hui Sun / Zhenghui Zha / Hongwei Yang / Qiongzi Huang / Dongqing Zhang / Zhenqin Chen / Xiangzhong Ye / Jinle Han / Lisheng Yang / Che Liu / Yuqiong Que / Mujin Fang / Ying Gu / Jun Zhang / Wenxin Luo / Z Hong Zhou / Shaowei Li / Tong Cheng / Ningshao Xia /   Abstract: Enterovirus uncoating receptors bind at the surface depression ("canyon") that encircles each capsid vertex causing the release of a host-derived lipid called "pocket factor" that is buried in a ...Enterovirus uncoating receptors bind at the surface depression ("canyon") that encircles each capsid vertex causing the release of a host-derived lipid called "pocket factor" that is buried in a hydrophobic pocket formed by the major viral capsid protein, VP1. Coxsackievirus and adenovirus receptor (CAR) is a universal uncoating receptor of group B coxsackieviruses (CVB). Here, we present five high-resolution cryoEM structures of CVB representing different stages of virus infection. Structural comparisons show that the CAR penetrates deeper into the canyon than other uncoating receptors, leading to a cascade of events: collapse of the VP1 hydrophobic pocket, high-efficiency release of the pocket factor and viral uncoating and genome release under neutral pH, as compared with low pH. Furthermore, we identified a potent therapeutic antibody that can neutralize viral infection by interfering with virion-CAR interactions, destabilizing the capsid and inducing virion disruption. Together, these results define the structural basis of CVB cell entry and antibody neutralization. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30815.map.gz emd_30815.map.gz | 633.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30815-v30.xml emd-30815-v30.xml emd-30815.xml emd-30815.xml | 19.2 KB 19.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30815.png emd_30815.png | 234.4 KB | ||
| Filedesc metadata |  emd-30815.cif.gz emd-30815.cif.gz | 6.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30815 http://ftp.pdbj.org/pub/emdb/structures/EMD-30815 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30815 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30815 | HTTPS FTP |
-Related structure data
| Related structure data |  7dq7MC  7dpfC  7dpgC  7dpzC  7dq1C  7dq4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30815.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30815.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.12 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Coxsackievirus B1 complex with nAb 5F5
+Supramolecule #1: Coxsackievirus B1 complex with nAb 5F5
+Supramolecule #2: Coxsackievirus B1
+Supramolecule #3: nAb 5F5
+Macromolecule #1: Virion protein 1
+Macromolecule #2: VP2
+Macromolecule #3: VP3
+Macromolecule #4: Capsid protein VP4
+Macromolecule #5: 5F5 VL
+Macromolecule #6: 5F5 VH
+Macromolecule #7: PALMITIC ACID
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















