+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Microsomal triglyceride transfer protein | |||||||||
 Map data Map data | Microsomal triglyceride transfer protein | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Microsomal triglyceride transfer protein / human liver / LIPID TRANSPORT / ISOMERASE / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationplasma lipoprotein particle assembly / triglyceride transfer activity / chylomicron assembly / peptidyl-proline hydroxylation to 4-hydroxy-L-proline / regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / phosphatidylcholine transfer activity / procollagen-proline 4-dioxygenase complex / triglyceride transport / insulin processing / phosphatidylethanolamine transfer activity ...plasma lipoprotein particle assembly / triglyceride transfer activity / chylomicron assembly / peptidyl-proline hydroxylation to 4-hydroxy-L-proline / regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / phosphatidylcholine transfer activity / procollagen-proline 4-dioxygenase complex / triglyceride transport / insulin processing / phosphatidylethanolamine transfer activity / VLDL assembly / thiol oxidase activity / procollagen-proline 4-dioxygenase activity / phospholipid transfer activity / LDL remodeling / ceramide 1-phosphate transfer activity / protein disulfide-isomerase / very-low-density lipoprotein particle assembly / endoplasmic reticulum chaperone complex / phospholipid transporter activity / protein folding in endoplasmic reticulum / lipoprotein transport / Collagen biosynthesis and modifying enzymes / lipid transporter activity / Chylomicron assembly / lipoprotein metabolic process / phospholipid transport / interleukin-23-mediated signaling pathway / cholesterol transfer activity / Interleukin-23 signaling / interleukin-12-mediated signaling pathway / low-density lipoprotein particle remodeling / Interleukin-12 signaling / cellular response to interleukin-7 / triglyceride metabolic process / Insulin processing / protein disulfide isomerase activity / Detoxification of Reactive Oxygen Species / microvillus membrane / endoplasmic reticulum-Golgi intermediate compartment / protein-disulfide reductase activity / protein secretion / apolipoprotein binding / endoplasmic reticulum to Golgi vesicle-mediated transport / positive regulation of T cell migration / positive regulation of substrate adhesion-dependent cell spreading / positive regulation of cell adhesion / cholesterol homeostasis / response to endoplasmic reticulum stress / Post-translational protein phosphorylation / establishment of localization in cell / lipid metabolic process / brush border membrane / Hedgehog ligand biogenesis / circadian rhythm / response to calcium ion / integrin binding / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / melanosome / lamellipodium / protein folding / actin binding / cellular response to hypoxia / basolateral plasma membrane / vesicle / cytoskeleton / positive regulation of viral entry into host cell / receptor complex / endoplasmic reticulum lumen / protein heterodimerization activity / external side of plasma membrane / focal adhesion / lipid binding / protein-containing complex binding / enzyme binding / endoplasmic reticulum / Golgi apparatus / protein-containing complex / RNA binding / extracellular exosome / extracellular region / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
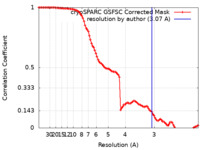
| Method | single particle reconstruction / cryo EM / Resolution: 3.07 Å | |||||||||
 Authors Authors | Zhang Z | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2023 Journal: Cell Rep / Year: 2023Title: High-resolution structural-omics of human liver enzymes. Authors: Chih-Chia Su / Meinan Lyu / Zhemin Zhang / Masaru Miyagi / Wei Huang / Derek J Taylor / Edward W Yu /  Abstract: We applied raw human liver microsome lysate to a holey carbon grid and used cryo-electron microscopy (cryo-EM) to define its composition. From this sample we identified and simultaneously determined ...We applied raw human liver microsome lysate to a holey carbon grid and used cryo-electron microscopy (cryo-EM) to define its composition. From this sample we identified and simultaneously determined high-resolution structural information for ten unique human liver enzymes involved in diverse cellular processes. Notably, we determined the structure of the endoplasmic bifunctional protein H6PD, where the N- and C-terminal domains independently possess glucose-6-phosphate dehydrogenase and 6-phosphogluconolactonase enzymatic activity, respectively. We also obtained the structure of heterodimeric human GANAB, an ER glycoprotein quality-control machinery that contains a catalytic α subunit and a noncatalytic β subunit. In addition, we observed a decameric peroxidase, PRDX4, which directly contacts a disulfide isomerase-related protein, ERp46. Structural data suggest that several glycosylations, bound endogenous compounds, and ions associate with these human liver enzymes. These results highlight the importance of cryo-EM in facilitating the elucidation of human organ proteomics at the atomic level. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28377.map.gz emd_28377.map.gz | 97.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28377-v30.xml emd-28377-v30.xml emd-28377.xml emd-28377.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28377_fsc.xml emd_28377_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_28377.png emd_28377.png | 96.3 KB | ||
| Filedesc metadata |  emd-28377.cif.gz emd-28377.cif.gz | 5.9 KB | ||
| Others |  emd_28377_additional_1.map.gz emd_28377_additional_1.map.gz emd_28377_half_map_1.map.gz emd_28377_half_map_1.map.gz emd_28377_half_map_2.map.gz emd_28377_half_map_2.map.gz | 52.1 MB 95.6 MB 95.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28377 http://ftp.pdbj.org/pub/emdb/structures/EMD-28377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28377 | HTTPS FTP |
-Related structure data
| Related structure data |  8eojMC  7uzmC  8ekwC  8ekyC  8em2C  8emrC  8emsC  8emtC  8eneC  8eorC  23426 M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28377.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28377.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Microsomal triglyceride transfer protein | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
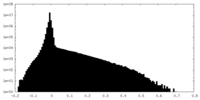
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Microsomal triglyceride transfer protein
| File | emd_28377_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Microsomal triglyceride transfer protein | ||||||||||||
| Projections & Slices |
| ||||||||||||
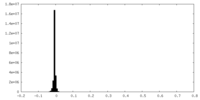
| Density Histograms |
-Half map: Microsomal triglyceride transfer protein
| File | emd_28377_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Microsomal triglyceride transfer protein | ||||||||||||
| Projections & Slices |
| ||||||||||||
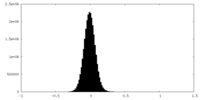
| Density Histograms |
-Half map: Microsomal triglyceride transfer protein
| File | emd_28377_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Microsomal triglyceride transfer protein | ||||||||||||
| Projections & Slices |
| ||||||||||||
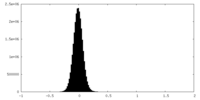
| Density Histograms |
- Sample components
Sample components
-Entire : Microsomal triglyceride transfer protein
| Entire | Name: Microsomal triglyceride transfer protein |
|---|---|
| Components |
|
-Supramolecule #1: Microsomal triglyceride transfer protein
| Supramolecule | Name: Microsomal triglyceride transfer protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Protein disulfide-isomerase
| Macromolecule | Name: Protein disulfide-isomerase / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: protein disulfide-isomerase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 57.190137 KDa |
| Sequence | String: MLRRALLCLA VAALVRADAP EEEDHVLVLR KSNFAEALAA HKYLLVEFYA PWCGHCKALA PEYAKAAGKL KAEGSEIRLA KVDATEESD LAQQYGVRGY PTIKFFRNGD TASPKEYTAG READDIVNWL KKRTGPAATT LPDGAAAESL VESSEVAVIG F FKDVESDS ...String: MLRRALLCLA VAALVRADAP EEEDHVLVLR KSNFAEALAA HKYLLVEFYA PWCGHCKALA PEYAKAAGKL KAEGSEIRLA KVDATEESD LAQQYGVRGY PTIKFFRNGD TASPKEYTAG READDIVNWL KKRTGPAATT LPDGAAAESL VESSEVAVIG F FKDVESDS AKQFLQAAEA IDDIPFGITS NSDVFSKYQL DKDGVVLFKK FDEGRNNFEG EVTKENLLDF IKHNQLPLVI EF TEQTAPK IFGGEIKTHI LLFLPKSVSD YDGKLSNFKT AAESFKGKIL FIFIDSDHTD NQRILEFFGL KKEECPAVRL ITL EEEMTK YKPESEELTA ERITEFCHRF LEGKIKPHLM SQELPEDWDK QPVKVLVGKN FEDVAFDEKK NVFVEFYAPW CGHC KQLAP IWDKLGETYK DHENIVIAKM DSTANEVEAV KVHSFPTLKF FPASADRTVI DYNGERTLDG FKKFLESGGQ DGAGD DDDL EDLEEAEEPD MEEDDDQKAV KDEL UniProtKB: Protein disulfide-isomerase |
-Macromolecule #2: Microsomal triglyceride transfer protein large subunit
| Macromolecule | Name: Microsomal triglyceride transfer protein large subunit type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 99.474102 KDa |
| Sequence | String: MILLAVLFLC FISSYSASVK GHTTGLSLNN DRLYKLTYST EVLLDRGKGK LQDSVGYRIS SNVDVALLWR NPDGDDDQLI QITMKDVNV ENVNQQRGEK SIFKGKSPSK IMGKENLEAL QRPTLLHLIH GKVKEFYSYQ NEAVAIENIK RGLASLFQTQ L SSGTTNEV ...String: MILLAVLFLC FISSYSASVK GHTTGLSLNN DRLYKLTYST EVLLDRGKGK LQDSVGYRIS SNVDVALLWR NPDGDDDQLI QITMKDVNV ENVNQQRGEK SIFKGKSPSK IMGKENLEAL QRPTLLHLIH GKVKEFYSYQ NEAVAIENIK RGLASLFQTQ L SSGTTNEV DISGNCKVTY QAHQDKVIKI KALDSCKIAR SGFTTPNQVL GVSSKATSVT TYKIEDSFVI AVLAEETHNF GL NFLQTIK GKIVSKQKLE LKTTEAGPRL MSGKQAAAII KAVDSKYTAI PIVGQVFQSH CKGCPSLSEL WRSTRKYLQP DNL SKAEAV RNFLAFIQHL RTAKKEEILQ ILKMENKEVL PQLVDAVTSA QTSDSLEAIL DFLDFKSDSS IILQERFLYA CGFA SHPNE ELLRALISKF KGSIGSSDIR ETVMIITGTL VRKLCQNEGC KLKAVVEAKK LILGGLEKAE KKEDTRMYLL ALKNA LLPE GIPSLLKYAE AGEGPISHLA TTALQRYDLP FITDEVKKTL NRIYHQNRKV HEKTVRTAAA AIILNNNPSY MDVKNI LLS IGELPQEMNK YMLAIVQDIL RFEMPASKIV RRVLKEMVAH NYDRFSRSGS SSAYTGYIER SPRSASTYSL DILYSGS GI LRRSNLNIFQ YIGKAGLHGS QVVIEAQGLE ALIAATPDEG EENLDSYAGM SAILFDVQLR PVTFFNGYSD LMSKMLSA S GDPISVVKGL ILLIDHSQEL QLQSGLKANI EVQGGLAIDI SGAMEFSLWY RESKTRVKNR VTVVITTDIT VDSSFVKAG LETSTETEAG LEFISTVQFS QYPFLVCMQM DKDEAPFRQF EKKYERLSTG RGYVSQKRKE SVLAGCEFPL HQENSEMCKV VFAPQPDST SSGWF UniProtKB: Microsomal triglyceride transfer protein large subunit |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 41.25 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.291 µm / Nominal defocus min: 0.17 µm / Nominal magnification: 81000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)