+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Aldehyde dehydrogenase 1 family member A1 from human liver | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | aldehyde dehydrogenase / human liver / OXIDOREDUCTASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationfructosamine catabolic process / 3-deoxyglucosone dehydrogenase activity / benzaldehyde dehydrogenase (NAD+) / acetaldehyde dehydrogenase (NAD+) activity / benzaldehyde dehydrogenase (NAD+) activity / aminobutyraldehyde dehydrogenase / gamma-aminobutyric acid biosynthetic process / aminobutyraldehyde dehydrogenase (NAD+) activity / retinal dehydrogenase / maintenance of lens transparency ...fructosamine catabolic process / 3-deoxyglucosone dehydrogenase activity / benzaldehyde dehydrogenase (NAD+) / acetaldehyde dehydrogenase (NAD+) activity / benzaldehyde dehydrogenase (NAD+) activity / aminobutyraldehyde dehydrogenase / gamma-aminobutyric acid biosynthetic process / aminobutyraldehyde dehydrogenase (NAD+) activity / retinal dehydrogenase / maintenance of lens transparency / Fructose catabolism / Ethanol oxidation / aldehyde metabolic process / RA biosynthesis pathway / aldehyde dehydrogenase (NAD+) / androgen binding / cellular detoxification of aldehyde / aldehyde dehydrogenase (NAD+) activity / retinal dehydrogenase (NAD+) activity / negative regulation of cold-induced thermogenesis / retinol metabolic process / retinoid metabolic process / GTPase activator activity / NAD binding / axon / synapse / extracellular exosome / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
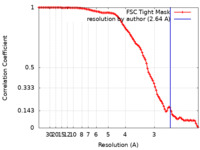
| Method | single particle reconstruction / cryo EM / Resolution: 2.64 Å | |||||||||
 Authors Authors | Zhang Z | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2023 Journal: Cell Rep / Year: 2023Title: High-resolution structural-omics of human liver enzymes. Authors: Chih-Chia Su / Meinan Lyu / Zhemin Zhang / Masaru Miyagi / Wei Huang / Derek J Taylor / Edward W Yu /  Abstract: We applied raw human liver microsome lysate to a holey carbon grid and used cryo-electron microscopy (cryo-EM) to define its composition. From this sample we identified and simultaneously determined ...We applied raw human liver microsome lysate to a holey carbon grid and used cryo-electron microscopy (cryo-EM) to define its composition. From this sample we identified and simultaneously determined high-resolution structural information for ten unique human liver enzymes involved in diverse cellular processes. Notably, we determined the structure of the endoplasmic bifunctional protein H6PD, where the N- and C-terminal domains independently possess glucose-6-phosphate dehydrogenase and 6-phosphogluconolactonase enzymatic activity, respectively. We also obtained the structure of heterodimeric human GANAB, an ER glycoprotein quality-control machinery that contains a catalytic α subunit and a noncatalytic β subunit. In addition, we observed a decameric peroxidase, PRDX4, which directly contacts a disulfide isomerase-related protein, ERp46. Structural data suggest that several glycosylations, bound endogenous compounds, and ions associate with these human liver enzymes. These results highlight the importance of cryo-EM in facilitating the elucidation of human organ proteomics at the atomic level. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28271.map.gz emd_28271.map.gz | 97.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28271-v30.xml emd-28271-v30.xml emd-28271.xml emd-28271.xml | 15.9 KB 15.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28271_fsc.xml emd_28271_fsc.xml | 11.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_28271.png emd_28271.png | 224.6 KB | ||
| Filedesc metadata |  emd-28271.cif.gz emd-28271.cif.gz | 5.3 KB | ||
| Others |  emd_28271_additional_1.map.gz emd_28271_additional_1.map.gz emd_28271_half_map_1.map.gz emd_28271_half_map_1.map.gz emd_28271_half_map_2.map.gz emd_28271_half_map_2.map.gz | 52 MB 95.3 MB 95.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28271 http://ftp.pdbj.org/pub/emdb/structures/EMD-28271 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28271 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28271 | HTTPS FTP |
-Related structure data
| Related structure data |  8eneMC  7uzmC  8ekwC  8ekyC  8em2C  8emrC  8emsC  8emtC  8eojC  8eorC  23428 M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28271.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28271.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
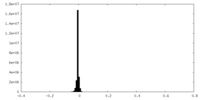
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_28271_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
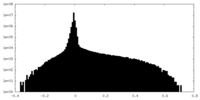
| Density Histograms |
-Half map: #2
| File | emd_28271_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
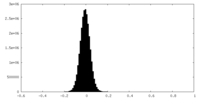
| Density Histograms |
-Half map: #1
| File | emd_28271_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
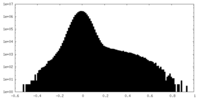
| Density Histograms |
- Sample components
Sample components
-Entire : Aldehyde dehydrogenase 1 family member A1
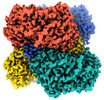
| Entire | Name: Aldehyde dehydrogenase 1 family member A1 |
|---|---|
| Components |
|
-Supramolecule #1: Aldehyde dehydrogenase 1 family member A1
| Supramolecule | Name: Aldehyde dehydrogenase 1 family member A1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Retinal dehydrogenase 1
| Macromolecule | Name: Retinal dehydrogenase 1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO EC number: Oxidoreductases; Acting on the aldehyde or oxo group of donors; With NAD+ or NADP+ as acceptor |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 54.924617 KDa |
| Sequence | String: MSSSGTPDLP VLLTDLKIQY TKIFINNEWH DSVSGKKFPV FNPATEEELC QVEEGDKEDV DKAVKAARQA FQIGSPWRTM DASERGRLL YKLADLIERD RLLLATMESM NGGKLYSNAY LNDLAGCIKT LRYCAGWADK IQGRTIPIDG NFFTYTRHEP I GVCGQIIP ...String: MSSSGTPDLP VLLTDLKIQY TKIFINNEWH DSVSGKKFPV FNPATEEELC QVEEGDKEDV DKAVKAARQA FQIGSPWRTM DASERGRLL YKLADLIERD RLLLATMESM NGGKLYSNAY LNDLAGCIKT LRYCAGWADK IQGRTIPIDG NFFTYTRHEP I GVCGQIIP WNFPLVMLIW KIGPALSCGN TVVVKPAEQT PLTALHVASL IKEAGFPPGV VNIVPGYGPT AGAAISSHMD ID KVAFTGS TEVGKLIKEA AGKSNLKRVT LELGGKSPCI VLADADLDNA VEFAHHGVFY HQGQCCIAAS RIFVEESIYD EFV RRSVER AKKYILGNPL TPGVTQGPQI DKEQYDKILD LIESGKKEGA KLECGGGPWG NKGYFVQPTV FSNVTDEMRI AKEE IFGPV QQIMKFKSLD DVIKRANNTF YGLSAGVFTK DIDKAITISS ALQAGTVWVN CYGVVSAQCP FGGFKMSGNG RELGE YGFH EYTEVKTVTV KISQKNS UniProtKB: Aldehyde dehydrogenase 1A1 |
-Macromolecule #2: NICOTINAMIDE-ADENINE-DINUCLEOTIDE
| Macromolecule | Name: NICOTINAMIDE-ADENINE-DINUCLEOTIDE / type: ligand / ID: 2 / Number of copies: 4 / Formula: NAD |
|---|---|
| Molecular weight | Theoretical: 663.425 Da |
| Chemical component information |  ChemComp-NAD: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 41.25 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.291 µm / Nominal defocus min: 0.17 µm / Nominal magnification: 81000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)