[English] 日本語
 Yorodumi
Yorodumi- EMDB-24220: Cryo-EM structure of ATP13A2 D458N/D962N mutant in the E1-apo sta... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24220 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of ATP13A2 D458N/D962N mutant in the E1-apo state, Conformation 1 | |||||||||
 Map data Map data | Summed, unsharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | P-type ATPase / P5B-ATPase / polyamine transporter / TRANSPORT PROTEIN | |||||||||
| Function / homology | Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate / Isoform 3 of Polyamine-transporting ATPase 13A2 Function and homology information Function and homology information | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
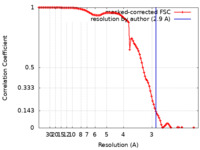
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Sim SI / Park E | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2021 Journal: Mol Cell / Year: 2021Title: Structural basis of polyamine transport by human ATP13A2 (PARK9). Authors: Sue Im Sim / Sören von Bülow / Gerhard Hummer / Eunyong Park /   Abstract: Polyamines are small, organic polycations that are ubiquitous and essential to all forms of life. Currently, how polyamines are transported across membranes is not understood. Recent studies have ...Polyamines are small, organic polycations that are ubiquitous and essential to all forms of life. Currently, how polyamines are transported across membranes is not understood. Recent studies have suggested that ATP13A2 and its close homologs, collectively known as P5B-ATPases, are polyamine transporters at endo-/lysosomes. Loss-of-function mutations of ATP13A2 in humans cause hereditary early-onset Parkinson's disease. To understand the polyamine transport mechanism of ATP13A2, we determined high-resolution cryoelectron microscopy (cryo-EM) structures of human ATP13A2 in five distinct conformational intermediates, which together, represent a near-complete transport cycle of ATP13A2. The structural basis of the polyamine specificity was revealed by an endogenous polyamine molecule bound to a narrow, elongated cavity within the transmembrane domain. The structures show an atypical transport path for a water-soluble substrate, in which polyamines may exit within the cytosolic leaflet of the membrane. Our study provides important mechanistic insights into polyamine transport and a framework to understand the functions and mechanisms of P5B-ATPases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24220.map.gz emd_24220.map.gz | 51.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24220-v30.xml emd-24220-v30.xml emd-24220.xml emd-24220.xml | 24.2 KB 24.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24220_fsc.xml emd_24220_fsc.xml | 10.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_24220.png emd_24220.png | 104.5 KB | ||
| Filedesc metadata |  emd-24220.cif.gz emd-24220.cif.gz | 6.8 KB | ||
| Others |  emd_24220_additional_1.map.gz emd_24220_additional_1.map.gz emd_24220_half_map_1.map.gz emd_24220_half_map_1.map.gz emd_24220_half_map_2.map.gz emd_24220_half_map_2.map.gz | 97.4 MB 95.6 MB 95.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24220 http://ftp.pdbj.org/pub/emdb/structures/EMD-24220 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24220 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24220 | HTTPS FTP |
-Validation report
| Summary document |  emd_24220_validation.pdf.gz emd_24220_validation.pdf.gz | 792.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24220_full_validation.pdf.gz emd_24220_full_validation.pdf.gz | 792.2 KB | Display | |
| Data in XML |  emd_24220_validation.xml.gz emd_24220_validation.xml.gz | 18.4 KB | Display | |
| Data in CIF |  emd_24220_validation.cif.gz emd_24220_validation.cif.gz | 23.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24220 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24220 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24220 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24220 | HTTPS FTP |
-Related structure data
| Related structure data |  7n75MC  7n70C  7n72C  7n73C  7n74C  7n76C  7n77C  7n78C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_24220.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24220.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
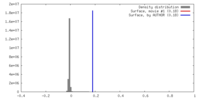
| Annotation | Summed, unsharpened map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.049 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Summed, sharpened map
| File | emd_24220_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
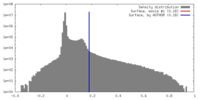
| Annotation | Summed, sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1
| File | emd_24220_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
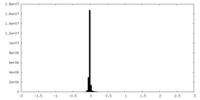
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2
| File | emd_24220_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
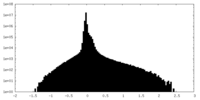
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human ATP13A2 D458N/D962N mutant
| Entire | Name: Human ATP13A2 D458N/D962N mutant |
|---|---|
| Components |
|
-Supramolecule #1: Human ATP13A2 D458N/D962N mutant
| Supramolecule | Name: Human ATP13A2 D458N/D962N mutant / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Isoform 3 of Polyamine-transporting ATPase 13A2
| Macromolecule | Name: Isoform 3 of Polyamine-transporting ATPase 13A2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number: Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 128.4425 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSADSSPLVG STPTGYGTLT IGTSIDPLSS SVSSVRLSGY CGSPWRVIGY HVVVWMMAGI PLLLFRWKPL WGVRLRLRPC NLAHAETLV IEIRDKEDSS WQLFTVQVQT EAIGEGSLEP SPQSQAEDGR SQAAVGAVPE GAWKDTAQLH KSEEAKRVLR Y YLFQGQRY ...String: MSADSSPLVG STPTGYGTLT IGTSIDPLSS SVSSVRLSGY CGSPWRVIGY HVVVWMMAGI PLLLFRWKPL WGVRLRLRPC NLAHAETLV IEIRDKEDSS WQLFTVQVQT EAIGEGSLEP SPQSQAEDGR SQAAVGAVPE GAWKDTAQLH KSEEAKRVLR Y YLFQGQRY IWIETQQAFY QVSLLDHGRS CDDVHRSRHG LSLQDQMVRK AIYGPNVISI PVKSYPQLLV DEALNPYYGF QA FSIALWL ADHYYWYALC IFLISSISIC LSLYKTRKQS QTLRDMVKLS MRVCVCRPGG EEEWVDSSEL VPGDCLVLPQ EGG LMPCDA ALVAGECMVN ESSLTGESIP VLKTALPEGL GPYCAETHRR HTLFCGTLIL QARAYVGPHV LAVVTRTGFC TAKG GLVSS ILHPRPINFK FYKHSMKFVA ALSVLALLGT IYSIFILYRN RVPLNEIVIR ALNLVTVVVP PALPAAMTVC TLYAQ SRLR RQGIFCIHPL RINLGGKLQL VCFDKTGTLT EDGLDVMGVV PLKGQAFLPL VPEPRRLPVG PLLRALATCH ALSRLQ DTP VGDPMDLKMV ESTGWVLEEE PAADSAFGTQ VLAVMRPPLW EPQLQAMEEP PVPVSVLHRF PFSSALQRMS VVVAWPG AT QPEAYVKGSP ELVAGLCNPE TVPTDFAQML QSYTAAGYRV VALASKPLPT VPSLEAAQQL TRDTVEGDLS LLGLLVMR N LLKPQTTPVI QALRRTRIRA VMVTGDNLQT AVTVARGCGM VAPQEHLIIV HATHPERGQP ASLEFLPMES PTAVNGVKD PDQAASYTVE PDPRSRHLAL SGPTFGIIVK HFPKLLPKVL VQGTVFARMA PEQKTELVCE LQKLQYCVGM CGDGANDCGA LKAADVGIS LSQAEASVVS PFTSSMASIE CVPMVIREGR CSLDTSFSVF KYMALYSLTQ FISVLILYTI NTNLGDLQFL A INLVITTT VAVLMSRTGP ALVLGRVRPP GALLSVPVLS SLLLQMVLVT GVQLGGYFLT LAQPWFVPLN RTVAAPDNLP NY ENTVVFS LSSFQYLILA AAVSKGAPFR RPLYTNVPFL VALALLSSVL VGLVLVPGLL QGPLALRNIT DTGFKLLLLG LVT LNFVGA FMLESVLDQC LPACLRRLRP KRASKKRFKQ LERELAEQPW PPLPAGPLR UniProtKB: Isoform 3 of Polyamine-transporting ATPase 13A2 |
-Macromolecule #2: water
| Macromolecule | Name: water / type: ligand / ID: 2 / Number of copies: 3 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 40 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)