+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24213 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of ATP13A2 in the E2-Pi state | |||||||||
 Map data Map data | Summed, unsharpened map | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationpolyamine transmembrane transport / spermine transmembrane transport / : / polyamine transmembrane transporter activity / ABC-type polyamine transporter activity / regulation of autophagosome size / extracellular exosome biogenesis / negative regulation of lysosomal protein catabolic process / regulation of chaperone-mediated autophagy / P-type ion transporter activity ...polyamine transmembrane transport / spermine transmembrane transport / : / polyamine transmembrane transporter activity / ABC-type polyamine transporter activity / regulation of autophagosome size / extracellular exosome biogenesis / negative regulation of lysosomal protein catabolic process / regulation of chaperone-mediated autophagy / P-type ion transporter activity / regulation of autophagy of mitochondrion / regulation of lysosomal protein catabolic process / intracellular monoatomic cation homeostasis / autophagosome-lysosome fusion / autophagosome organization / protein localization to lysosome / positive regulation of exosomal secretion / phosphatidic acid binding / ATPase-coupled monoatomic cation transmembrane transporter activity / multivesicular body membrane / intracellular zinc ion homeostasis / cupric ion binding / regulation of protein localization to nucleus / regulation of mitochondrion organization / phosphatidylinositol-3,5-bisphosphate binding / Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate / lysosomal transport / regulation of intracellular protein transport / lipid homeostasis / cellular response to zinc ion / autophagosome membrane / Ion transport by P-type ATPases / regulation of macroautophagy / transport vesicle / cellular response to manganese ion / multivesicular body / lysosomal lumen / autophagosome / regulation of neuron apoptotic process / positive regulation of protein secretion / autophagy / intracellular calcium ion homeostasis / late endosome / late endosome membrane / manganese ion binding / cellular response to oxidative stress / monoatomic ion transmembrane transport / vesicle / intracellular iron ion homeostasis / lysosome / neuron projection / lysosomal membrane / neuronal cell body / positive regulation of gene expression / ATP hydrolysis activity / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
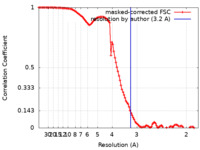
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Sim SI / Park E | |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2021 Journal: Mol Cell / Year: 2021Title: Structural basis of polyamine transport by human ATP13A2 (PARK9). Authors: Sue Im Sim / Sören von Bülow / Gerhard Hummer / Eunyong Park /   Abstract: Polyamines are small, organic polycations that are ubiquitous and essential to all forms of life. Currently, how polyamines are transported across membranes is not understood. Recent studies have ...Polyamines are small, organic polycations that are ubiquitous and essential to all forms of life. Currently, how polyamines are transported across membranes is not understood. Recent studies have suggested that ATP13A2 and its close homologs, collectively known as P5B-ATPases, are polyamine transporters at endo-/lysosomes. Loss-of-function mutations of ATP13A2 in humans cause hereditary early-onset Parkinson's disease. To understand the polyamine transport mechanism of ATP13A2, we determined high-resolution cryoelectron microscopy (cryo-EM) structures of human ATP13A2 in five distinct conformational intermediates, which together, represent a near-complete transport cycle of ATP13A2. The structural basis of the polyamine specificity was revealed by an endogenous polyamine molecule bound to a narrow, elongated cavity within the transmembrane domain. The structures show an atypical transport path for a water-soluble substrate, in which polyamines may exit within the cytosolic leaflet of the membrane. Our study provides important mechanistic insights into polyamine transport and a framework to understand the functions and mechanisms of P5B-ATPases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24213.map.gz emd_24213.map.gz | 62.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24213-v30.xml emd-24213-v30.xml emd-24213.xml emd-24213.xml | 19.9 KB 19.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24213_fsc.xml emd_24213_fsc.xml | 11.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_24213.png emd_24213.png | 56.9 KB | ||
| Others |  emd_24213_additional_1.map.gz emd_24213_additional_1.map.gz emd_24213_half_map_1.map.gz emd_24213_half_map_1.map.gz emd_24213_half_map_2.map.gz emd_24213_half_map_2.map.gz | 118 MB 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24213 http://ftp.pdbj.org/pub/emdb/structures/EMD-24213 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24213 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24213 | HTTPS FTP |
-Related structure data
| Related structure data |  7n70C  7n72C  7n73C  7n74C  7n75C  7n76C  7n77C  7n78C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24213.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24213.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Summed, unsharpened map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.911 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
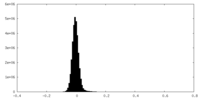
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Summed, sharpened map
| File | emd_24213_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Summed, sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
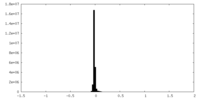
| Density Histograms |
-Half map: Half map 1
| File | emd_24213_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
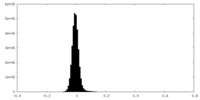
| Density Histograms |
-Half map: Half map 2
| File | emd_24213_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
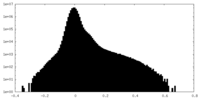
| Density Histograms |
- Sample components
Sample components
-Entire : Human ATP13A2 complexed with Pi
| Entire | Name: Human ATP13A2 complexed with Pi |
|---|---|
| Components |
|
-Supramolecule #1: Human ATP13A2 complexed with Pi
| Supramolecule | Name: Human ATP13A2 complexed with Pi / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
-Macromolecule #1: Human ATP13A2 (PARK9)
| Macromolecule | Name: Human ATP13A2 (PARK9) / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) Spodoptera aff. frugiperda 1 BOLD-2017 (butterflies/moths) |
| Sequence | String: MSADSSPLVG STPTGYGTLT IGTSIDPLSS SVSSVRLSGY CGSPWRVIGY HVVVWMMAGI PLLLFRWKPL WGVRLRLRP CNLAHAETLV IEIRDKEDSS WQLFTVQVQT EAIGEGSLEP SPQSQAEDGR SQAAVGAVPE G AWKDTAQL HKSEEAKRVL RYYLFQGQRY ...String: MSADSSPLVG STPTGYGTLT IGTSIDPLSS SVSSVRLSGY CGSPWRVIGY HVVVWMMAGI PLLLFRWKPL WGVRLRLRP CNLAHAETLV IEIRDKEDSS WQLFTVQVQT EAIGEGSLEP SPQSQAEDGR SQAAVGAVPE G AWKDTAQL HKSEEAKRVL RYYLFQGQRY IWIETQQAFY QVSLLDHGRS CDDVHRSRHG LSLQDQMVRK AI YGPNVIS IPVKSYPQLL VDEALNPYYG FQAFSIALWL ADHYYWYALC IFLISSISIC LSLYKTRKQS QTL RDMVKL SMRVCVCRPG GEEEWVDSSE LVPGDCLVLP QEGGLMPCDA ALVAGECMVN ESSLTGESIP VLKT ALPEG LGPYCAETHR RHTLFCGTLI LQARAYVGPH VLAVVTRTGF CTAKGGLVSS ILHPRPINFK FYKHS MKFV AALSVLALLG TIYSIFILYR NRVPLNEIVI RALDLVTVVV PPALPAAMTV CTLYAQSRLR RQGIFC IHP LRINLGGKLQ LVCFDKTGTL TEDGLDVMGV VPLKGQAFLP LVPEPRRLPV GPLLRALATC HALSRLQ DT PVGDPMDLKM VESTGWVLEE EPAADSAFGT QVLAVMRPPL WEPQLQAMEE PPVPVSVLHR FPFSSALQ R MSVVVAWPGA TQPEAYVKGS PELVAGLCNP ETVPTDFAQM LQSYTAAGYR VVALASKPLP TVPSLEAAQ QLTRDTVEGD LSLLGLLVMR NLLKPQTTPV IQALRRTRIR AVMVTGDNLQ TAVTVARGCG MVAPQEHLII VHATHPERG QPASLEFLPM ESPTAVNGVK DPDQAASYTV EPDPRSRHLA LSGPTFGIIV KHFPKLLPKV L VQGTVFAR MAPEQKTELV CELQKLQYCV GMCGDGANDC GALKAADVGI SLSQAEASVV SPFTSSMASI EC VPMVIRE GRCSLDTSFS VFKYMALYSL TQFISVLILY TINTNLGDLQ FLAIDLVITT TVAVLMSRTG PAL VLGRVR PPGALLSVPV LSSLLLQMVL VTGVQLGGYF LTLAQPWFVP LNRTVAAPDN LPNYENTVVF SLSS FQYLI LAAAVSKGAP FRRPLYTNVP FLVALALLSS VLVGLVLVPG LLQGPLALRN ITDTGFKLLL LGLVT LNFV GAFMLESVLD QCLPACLRRL RPKRASKKRF KQLERELAEQ PWPPLPAGPL R |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)