[English] 日本語
 Yorodumi
Yorodumi- EMDB-2377: The structure and super-organization of acetylcholine receptor-ra... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2377 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The structure and super-organization of acetylcholine receptor-rapsyn complexes; class A | |||||||||
 Map data Map data | Acetylcholine receptor with one rapsyn bound | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | neurotransmitter receptor / clustering / synapse / neuromuscular junction / nicotinic / acetylcholine receptor / rapsyn / 43K / electric organ | |||||||||
| Function / homology |  Function and homology information Function and homology informationprotein binding / acetylcholine-gated channel complex / acetylcholine-gated monoatomic cation-selective channel activity / acetylcholine receptor binding / acetylcholine receptor signaling pathway / transmembrane signaling receptor activity / cell junction / chemical synaptic transmission / cytoskeleton / postsynaptic membrane ...protein binding / acetylcholine-gated channel complex / acetylcholine-gated monoatomic cation-selective channel activity / acetylcholine receptor binding / acetylcholine receptor signaling pathway / transmembrane signaling receptor activity / cell junction / chemical synaptic transmission / cytoskeleton / postsynaptic membrane / synapse / zinc ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | subtomogram averaging / cryo EM / negative staining / Resolution: 41.0 Å | |||||||||
 Authors Authors | Zuber B / Unwin N | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2013 Journal: Proc Natl Acad Sci U S A / Year: 2013Title: Structure and superorganization of acetylcholine receptor-rapsyn complexes. Authors: Benoît Zuber / Nigel Unwin /  Abstract: The scaffolding protein at the neuromuscular junction, rapsyn, enables clustering of nicotinic acetylcholine receptors in high concentration and is critical for muscle function. Patients with ...The scaffolding protein at the neuromuscular junction, rapsyn, enables clustering of nicotinic acetylcholine receptors in high concentration and is critical for muscle function. Patients with insufficient receptor clustering suffer from muscle weakness. However, the detailed organization of the receptor-rapsyn network is poorly understood: it is unclear whether rapsyn first forms a wide meshwork to which receptors can subsequently dock or whether it only forms short bridges linking receptors together to make a large cluster. Furthermore, the number of rapsyn-binding sites per receptor (a heteropentamer) has been controversial. Here, we show by cryoelectron tomography and subtomogram averaging of Torpedo postsynaptic membrane that receptors are connected by up to three rapsyn bridges, the minimum number required to form a 2D network. Half of the receptors belong to rapsyn-connected groups comprising between two and fourteen receptors. Our results provide a structural basis for explaining the stability and low diffusion of receptors within clusters. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2377.map.gz emd_2377.map.gz | 423.7 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2377-v30.xml emd-2377-v30.xml emd-2377.xml emd-2377.xml | 14.6 KB 14.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_2377.tif emd_2377.tif | 129.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2377 http://ftp.pdbj.org/pub/emdb/structures/EMD-2377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2377 | HTTPS FTP |
-Related structure data
| Related structure data |  4boiMC 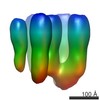 2376C  2378C 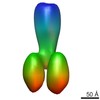 2381C  2382C 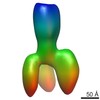 2383C  4bogC  4bonC  4booC  4borC  4botC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_2377.map.gz / Format: CCP4 / Size: 825.2 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2377.map.gz / Format: CCP4 / Size: 825.2 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Acetylcholine receptor with one rapsyn bound | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 7.48 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Postsynaptic membrane isolated from Torpedo marmorata electric organ
| Entire | Name: Postsynaptic membrane isolated from Torpedo marmorata electric organ |
|---|---|
| Components |
|
-Supramolecule #1000: Postsynaptic membrane isolated from Torpedo marmorata electric organ
| Supramolecule | Name: Postsynaptic membrane isolated from Torpedo marmorata electric organ type: sample / ID: 1000 / Details: class average Oligomeric state: acetylcholine receptors bound to one rapsyn Number unique components: 2 |
|---|---|
| Molecular weight | Theoretical: 336 KDa |
-Macromolecule #1: nicotinic acetylcholine receptor
| Macromolecule | Name: nicotinic acetylcholine receptor / type: protein_or_peptide / ID: 1 / Name.synonym: AChR / Number of copies: 1 / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 290 KDa / Theoretical: 280 KDa |
| Sequence | InterPro: Nicotinic acetylcholine receptor |
-Macromolecule #2: Rapsyn
| Macromolecule | Name: Rapsyn / type: protein_or_peptide / ID: 2 Name.synonym: 43 kDa receptor-associated protein of the synapse Details: the number of copies is suggested by the volume of the rapsyn density Number of copies: 1 / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 43 KDa / Theoretical: 46 KDa |
| Sequence | GO: chemical synaptic transmission, protein binding, zinc ion binding, acetylcholine receptor binding, cytoskeleton, cell junction, synapse, postsynaptic membrane InterPro: 43kDa postsynaptic protein, Rapsyn, myristoylation/linker region, N-terminal, Tetratricopeptide-like helical domain superfamily, INTERPRO: IPR013026, Zinc finger, RING/FYVE/PHD-type, Zinc ...InterPro: 43kDa postsynaptic protein, Rapsyn, myristoylation/linker region, N-terminal, Tetratricopeptide-like helical domain superfamily, INTERPRO: IPR013026, Zinc finger, RING/FYVE/PHD-type, Zinc finger, RING-type, Tetratricopeptide repeat, Tetratricopeptide repeat 1, 43kDa postsynaptic, conserved site |
-Experimental details
-Structure determination
| Method | negative staining, cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Details: 400 mM NaCl, 20 mM phosphate buffer, leupeptin 0.3 mg/l, pepstatin 1 mg/l |
|---|---|
| Staining | Type: NEGATIVE / Details: unstained |
| Grid | Details: 300 mesh copper grid with lacy carbon support, glow discharge in amylamine atmosphere |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 78 K / Instrument: HOMEMADE PLUNGER / Method: blot from the carbon side |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Temperature | Min: 80 K / Max: 95 K / Average: 85 K |
| Alignment procedure | Legacy - Astigmatism: objective lens astigmatism was corrected at 69,000 times magnification |
| Specialist optics | Energy filter - Name: Gatan / Energy filter - Lower energy threshold: 0.0 eV / Energy filter - Upper energy threshold: 20.0 eV |
| Details | dual tilt axis tomography |
| Date | Jul 25, 2008 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Digitization - Sampling interval: 15 µm / Number real images: 142 / Average electron dose: 50 e/Å2 / Details: dual axis tilt series / Bits/pixel: 16 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 80213 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 6.0 µm / Nominal defocus min: 3.0 µm / Nominal magnification: 69000 |
| Sample stage | Specimen holder model: GATAN LIQUID NITROGEN / Tilt series - Axis1 - Min angle: -70 ° / Tilt series - Axis1 - Max angle: 70 ° |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing #1
Image processing #1
| Image processing ID | 1 |
|---|---|
| Final reconstruction | Algorithm: OTHER / Software - Name: IMOD, PRIISM/IVE / Number subtomograms used: 3564 |
| Final 3D classification | Number classes: 5 |
- Image processing #2
Image processing #2
| Image processing ID | 2 |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 41.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: XMIPP, ML, TOMO / Number subtomograms used: 3564 |
| Final 3D classification | Number classes: 5 |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - #0 - Chain ID: A / Chain - #1 - Chain ID: B / Chain - #2 - Chain ID: C / Chain - #3 - Chain ID: D / Chain - #4 - Chain ID: E |
|---|---|
| Software | Name:  Chimera Chimera |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-4boi: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















