[English] 日本語
 Yorodumi
Yorodumi- EMDB-21901: CryoEM structure of Burkholderia pseudomallei hopanoid biosynthes... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21901 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of Burkholderia pseudomallei hopanoid biosynthesis-associated RND transporter HpnN | |||||||||
 Map data Map data | density modification in Phenix | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | CryoEM structure / hopanoid transporter HpnN / PROTEIN TRANSPORT | |||||||||
| Function / homology | Hopanoid biosynthesis associated RND transporter-like protein HpnN / Membrane transport protein MMPL domain / MMPL family / Sterol-sensing domain (SSD) profile. / Sterol-sensing domain / membrane => GO:0016020 / plasma membrane / Hopanoid biosynthesis associated RND transporter like protein HpnN Function and homology information Function and homology information | |||||||||
| Biological species |   Burkholderia pseudomallei MSHR346 (bacteria) Burkholderia pseudomallei MSHR346 (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.59 Å | |||||||||
 Authors Authors | Lyu M / Yu EW | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Methods / Year: 2021 Journal: Nat Methods / Year: 2021Title: A 'Build and Retrieve' methodology to simultaneously solve cryo-EM structures of membrane proteins. Authors: Chih-Chia Su / Meinan Lyu / Christopher E Morgan / Jani Reddy Bolla / Carol V Robinson / Edward W Yu /   Abstract: Single-particle cryo-electron microscopy (cryo-EM) has become a powerful technique in the field of structural biology. However, the inability to reliably produce pure, homogeneous membrane protein ...Single-particle cryo-electron microscopy (cryo-EM) has become a powerful technique in the field of structural biology. However, the inability to reliably produce pure, homogeneous membrane protein samples hampers the progress of their structural determination. Here, we develop a bottom-up iterative method, Build and Retrieve (BaR), that enables the identification and determination of cryo-EM structures of a variety of inner and outer membrane proteins, including membrane protein complexes of different sizes and dimensions, from a heterogeneous, impure protein sample. We also use the BaR methodology to elucidate structural information from Escherichia coli K12 crude membrane and raw lysate. The findings demonstrate that it is possible to solve high-resolution structures of a number of relatively small (<100 kDa) and less abundant (<10%) unidentified membrane proteins within a single, heterogeneous sample. Importantly, these results highlight the potential of cryo-EM for systems structural proteomics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21901.map.gz emd_21901.map.gz | 2.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21901-v30.xml emd-21901-v30.xml emd-21901.xml emd-21901.xml | 14.3 KB 14.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_21901.png emd_21901.png | 106.3 KB | ||
| Filedesc metadata |  emd-21901.cif.gz emd-21901.cif.gz | 6.1 KB | ||
| Others |  emd_21901_additional_1.map.gz emd_21901_additional_1.map.gz | 97.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21901 http://ftp.pdbj.org/pub/emdb/structures/EMD-21901 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21901 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21901 | HTTPS FTP |
-Validation report
| Summary document |  emd_21901_validation.pdf.gz emd_21901_validation.pdf.gz | 405.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_21901_full_validation.pdf.gz emd_21901_full_validation.pdf.gz | 404.8 KB | Display | |
| Data in XML |  emd_21901_validation.xml.gz emd_21901_validation.xml.gz | 4.2 KB | Display | |
| Data in CIF |  emd_21901_validation.cif.gz emd_21901_validation.cif.gz | 4.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21901 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21901 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21901 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21901 | HTTPS FTP |
-Related structure data
| Related structure data |  6wu0MC  6wtiC  6wtzC  6wu6C  7jz2C  7jz3C  7jz6C  7jzhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21901.map.gz / Format: CCP4 / Size: 2.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21901.map.gz / Format: CCP4 / Size: 2.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | density modification in Phenix | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
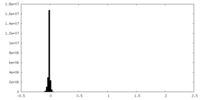
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: orignal in cryosparc
| File | emd_21901_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | orignal in cryosparc | ||||||||||||
| Projections & Slices |
| ||||||||||||
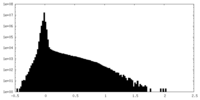
| Density Histograms |
- Sample components
Sample components
-Entire : hopanoid transporter HpnN
| Entire | Name: hopanoid transporter HpnN |
|---|---|
| Components |
|
-Supramolecule #1: hopanoid transporter HpnN
| Supramolecule | Name: hopanoid transporter HpnN / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Hopanoid biosynthesis associated RND transporter like protein HpnN
| Macromolecule | Name: Hopanoid biosynthesis associated RND transporter like protein HpnN type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Burkholderia pseudomallei MSHR346 (bacteria) Burkholderia pseudomallei MSHR346 (bacteria) |
| Molecular weight | Theoretical: 92.60093 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLTSVLVRLV AWSVRRPIWV VVLSLVIAAL SSVYVAHHFK INTDISKLVE NDPKWAALGR AIDDAFPQRN QTILAVVEAP APEFAGAAA DALAEGLRRE TEAGRIGQVS EPAGGPLFEH DGLLFLPEQD VATTTAQLAS ARPLINVLAK DPSIAGLATT L STTLGVPL ...String: MLTSVLVRLV AWSVRRPIWV VVLSLVIAAL SSVYVAHHFK INTDISKLVE NDPKWAALGR AIDDAFPQRN QTILAVVEAP APEFAGAAA DALAEGLRRE TEAGRIGQVS EPAGGPLFEH DGLLFLPEQD VATTTAQLAS ARPLINVLAK DPSIAGLATT L STTLGVPL QSGQVKLSGM AKLLSRSAAT VDDVLAGKPA AFSWRALVDA DAAREPARAF VTVQPVVNYG ALKAGEQASR TI RATAQAL KLDERFGAAV RLTGEQPLAD EEFASVQDGA LVNGIATLAI VLVILWIALR SKRMIASVFV TLFVGLVVTA ALG LMMVGS LNMISVAFMV LFVGLGVDFA IQYGVKYREE RHRDPNLDHA LVGAAHAMGM PLTLATAAVA ASFFSFLPTA YRGV SELGL IAGVGMFVAL FTTLTLLPAL LKLLAPPGER KPPGFPRLAP VDDYLDHHRK PILIGTLAVV IGALPLLAHL RFDFN PLHL KDPRSESMAT LLALKDSPEA SVNDVSLLAP SLVAANAAAQ RLGALPEVGR TTTLSTFIPD AQPQKLATIA AAARGL LPA LTQPAAAPVP DAQRVAALKR ASNLLEYASE DYPGPGAAAA KHLSESLAKL AAADAATRER AEHAFSVPLK IALNQLA ML LQPLEITREN LPPQIVRDWI APDGRALVQI SPKVVKGADP GDDAMLRRFA KAVKAAEPGA IGGPISILHS ADTIIRAF L QAAALSVVSI TVLLWITLRR FGDVLRTLVP LLVSGVVTLE LCVLLGMPLN FANIIALPLM LGVGVAFKVY FVMAWRAGQ TGLLQSSLTH AVLFSAATTA TAFGSLWLSH HPGTASMGRL LALALSCTLI GAVVFQPVLM GKPRTKRVTN QSQGIDE UniProtKB: Hopanoid biosynthesis associated RND transporter like protein HpnN |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)