+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20442 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human NatE complex (NatA/Naa50) | |||||||||
 Map data Map data | NatE complex (NatA/Naa50) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | NatA / Naa50 / NatE / TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of maintenance of mitotic sister chromatid cohesion, centromeric / mitotic sister chromatid cohesion, centromeric / N-terminal methionine Nalpha-acetyltransferase NatE / protein-N-terminal-glutamate acetyltransferase activity / N-terminal amino-acid Nalpha-acetyltransferase NatA / N-terminal protein amino acid acetylation / NatA complex / protein N-terminal-methionine acetyltransferase activity / protein N-terminal-serine acetyltransferase activity / protein-N-terminal-alanine acetyltransferase activity ...negative regulation of maintenance of mitotic sister chromatid cohesion, centromeric / mitotic sister chromatid cohesion, centromeric / N-terminal methionine Nalpha-acetyltransferase NatE / protein-N-terminal-glutamate acetyltransferase activity / N-terminal amino-acid Nalpha-acetyltransferase NatA / N-terminal protein amino acid acetylation / NatA complex / protein N-terminal-methionine acetyltransferase activity / protein N-terminal-serine acetyltransferase activity / protein-N-terminal-alanine acetyltransferase activity / protein-N-terminal amino-acid acetyltransferase activity / internal protein amino acid acetylation / histone H4 acetyltransferase activity / N-acetyltransferase activity / establishment of mitotic sister chromatid cohesion / acetyltransferase activator activity / mitotic sister chromatid cohesion / protein acetylation / protein-lysine-acetyltransferase activity / chromosome organization / Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / post-translational protein modification / ribosome binding / angiogenesis / transcription regulator complex / cell differentiation / protein stabilization / nuclear body / intracellular membrane-bounded organelle / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / nucleolus / RNA binding / extracellular exosome / membrane / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.02 Å | |||||||||
 Authors Authors | Deng S / Marmorstein R | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Molecular basis for N-terminal acetylation by human NatE and its modulation by HYPK. Authors: Sunbin Deng / Nina McTiernan / Xuepeng Wei / Thomas Arnesen / Ronen Marmorstein /   Abstract: The human N-terminal acetyltransferase E (NatE) contains NAA10 and NAA50 catalytic, and NAA15 auxiliary subunits and associates with HYPK, a protein with intrinsic NAA10 inhibitory activity. NatE co- ...The human N-terminal acetyltransferase E (NatE) contains NAA10 and NAA50 catalytic, and NAA15 auxiliary subunits and associates with HYPK, a protein with intrinsic NAA10 inhibitory activity. NatE co-translationally acetylates the N-terminus of half the proteome to mediate diverse biological processes, including protein half-life, localization, and interaction. The molecular basis for how NatE and HYPK cooperate is unknown. Here, we report the cryo-EM structures of human NatE and NatE/HYPK complexes and associated biochemistry. We reveal that NAA50 and HYPK exhibit negative cooperative binding to NAA15 in vitro and in human cells by inducing NAA15 shifts in opposing directions. NAA50 and HYPK each contribute to NAA10 activity inhibition through structural alteration of the NAA10 substrate-binding site. NAA50 activity is increased through NAA15 tethering, but is inhibited by HYPK through structural alteration of the NatE substrate-binding site. These studies reveal the molecular basis for coordinated N-terminal acetylation by NatE and HYPK. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20442.map.gz emd_20442.map.gz | 7.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20442-v30.xml emd-20442-v30.xml emd-20442.xml emd-20442.xml | 16.1 KB 16.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20442.png emd_20442.png | 133 KB | ||
| Filedesc metadata |  emd-20442.cif.gz emd-20442.cif.gz | 6.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20442 http://ftp.pdbj.org/pub/emdb/structures/EMD-20442 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20442 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20442 | HTTPS FTP |
-Related structure data
| Related structure data |  6pplMC  6pw9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20442.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20442.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
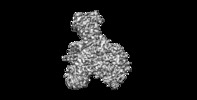
| Annotation | NatE complex (NatA/Naa50) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.832 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human NatE complex
| Entire | Name: human NatE complex |
|---|---|
| Components |
|
-Supramolecule #1: human NatE complex
| Supramolecule | Name: human NatE complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: N-alpha-acetyltransferase 50
| Supramolecule | Name: N-alpha-acetyltransferase 50 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: N-alpha-acetyltransferase 15, NatA auxiliary subunit, N-alpha-ace...
| Supramolecule | Name: N-alpha-acetyltransferase 15, NatA auxiliary subunit, N-alpha-acetyltransferase 10 type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: N-alpha-acetyltransferase 50
| Macromolecule | Name: N-alpha-acetyltransferase 50 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number: N-terminal methionine Nalpha-acetyltransferase NatE |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 19.427373 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKGSRIELGD VTPHNIKQLK RLNQVIFPVS YNDKFYKDVL EVGELAKLAY FNDIAVGAVC CRVDHSQNQK RLYIMTLGCL APYRRLGIG TKMLNHVLNI CEKDGTFDNI YLHVQISNES AIDFYRKFGF EIIETKKNYY KRIEPADAHV LQKNLKVPSG Q NADVQKTD N UniProtKB: N-alpha-acetyltransferase 50 |
-Macromolecule #2: N-alpha-acetyltransferase 15, NatA auxiliary subunit
| Macromolecule | Name: N-alpha-acetyltransferase 15, NatA auxiliary subunit / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 101.427562 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPAVSLPPKE NALFKRILRC YEHKQYRNGL KFCKQILSNP KFAEHGETLA MKGLTLNCLG KKEEAYELVR RGLRNDLKSH VCWHVYGLL QRSDKKYDEA IKCYRNALKW DKDNLQILRD LSLLQIQMRD LEGYRETRYQ LLQLRPAQRA SWIGYAIAYH L LEDYEMAA ...String: MPAVSLPPKE NALFKRILRC YEHKQYRNGL KFCKQILSNP KFAEHGETLA MKGLTLNCLG KKEEAYELVR RGLRNDLKSH VCWHVYGLL QRSDKKYDEA IKCYRNALKW DKDNLQILRD LSLLQIQMRD LEGYRETRYQ LLQLRPAQRA SWIGYAIAYH L LEDYEMAA KILEEFRKTQ QTSPDKVDYE YSELLLYQNQ VLREAGLYRE ALEHLCTYEK QICDKLAVEE TKGELLLQLC RL EDAADVY RGLQERNPEN WAYYKGLEKA LKPANMLERL KIYEEAWTKY PRGLVPRRLP LNFLSGEKFK ECLDKFLRMN FSK GCPPVF NTLRSLYKDK EKVAIIEELV VGYETSLKSC RLFNPNDDGK EEPPTTLLWV QYYLAQHYDK IGQPSIALEY INTA IESTP TLIELFLVKA KIYKHAGNIK EAARWMDEAQ ALDTADRFIN SKCAKYMLKA NLIKEAEEMC SKFTREGTSA VENLN EMQC MWFQTECAQA YKAMNKFGEA LKKCHEIERH FIEITDDQFD FHTYCMRKIT LRSYVDLLKL EDVLRQHPFY FKAARI AIE IYLKLHDNPL TDENKEHEAD TANMSDKELK KLRNKQRRAQ KKAQIEEEKK NAEKEKQQRN QKKKKDDDDE EIGGPKE EL IPEKLAKVET PLEEAIKFLT PLKNLVKNKI ETHLFAFEIY FRKEKFLLML QSVKRAFAID SSHPWLHECM IRLFNTAV C ESKDLSDTVR TVLKQEMNRL FGATNPKNFN ETFLKRNSDS LPHRLSAAKM VYYLDPSSQK RAIELATTLD ESLTNRNLQ TCMEVLEALY DGSLGDCKEA AEIYRANCHK LFPYALAFMP PGYEEDMKIT VNGDSSAEAE ELANEI UniProtKB: N-alpha-acetyltransferase 15, NatA auxiliary subunit |
-Macromolecule #3: N-alpha-acetyltransferase 10
| Macromolecule | Name: N-alpha-acetyltransferase 10 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO EC number: N-terminal amino-acid Nalpha-acetyltransferase NatA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 26.522602 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: (ACE)MNIRNARPE DLMNMQHCNL LCLPENYQMK YYFYHGLSWP QLSYIAEDEN GKIVGYVLAK MEEDPDDVPH GHITSL AVK RSHRRLGLAQ KLMDQASRAM IENFNAKYVS LHVRKSNRAA LHLYSNTLNF QISEVEPKYY ADGEDAYAMK RDLTQMA DE LRRHLELKEK ...String: (ACE)MNIRNARPE DLMNMQHCNL LCLPENYQMK YYFYHGLSWP QLSYIAEDEN GKIVGYVLAK MEEDPDDVPH GHITSL AVK RSHRRLGLAQ KLMDQASRAM IENFNAKYVS LHVRKSNRAA LHLYSNTLNF QISEVEPKYY ADGEDAYAMK RDLTQMA DE LRRHLELKEK GRHVVLGAIE NKVESKGNSP PSSGEACREE KGLAAEDSGG DSKDLSEVSE TTESTDVKDS SEASDSAS UniProtKB: N-alpha-acetyltransferase 10 |
-Macromolecule #4: ACETYL COENZYME *A
| Macromolecule | Name: ACETYL COENZYME *A / type: ligand / ID: 4 / Number of copies: 2 / Formula: ACO |
|---|---|
| Molecular weight | Theoretical: 809.571 Da |
| Chemical component information |  ChemComp-ACO: |
-Macromolecule #5: INOSITOL HEXAKISPHOSPHATE
| Macromolecule | Name: INOSITOL HEXAKISPHOSPHATE / type: ligand / ID: 5 / Number of copies: 1 / Formula: IHP |
|---|---|
| Molecular weight | Theoretical: 660.035 Da |
| Chemical component information |  ChemComp-IHP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 289 K / Instrument: FEI VITROBOT MARK II |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 1.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.02 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 353541 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)