[English] 日本語
 Yorodumi
Yorodumi- EMDB-20217: Structure of SIVsmm Nef and SMM tetherin bound to the clathrin ad... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20217 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
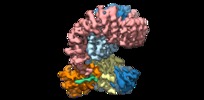
| Title | Structure of SIVsmm Nef and SMM tetherin bound to the clathrin adaptor AP-2 complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AP / HIV / Nef / trafficking / Protein transport / viral restriction factor | |||||||||
| Function / homology |  Function and homology information Function and homology informationAP-type membrane coat adaptor complex / Formation of annular gap junctions / Gap junction degradation / LDL clearance / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / WNT5A-dependent internalization of FZD4 / clathrin coat / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / Trafficking of GluR2-containing AMPA receptors / LDL clearance ...AP-type membrane coat adaptor complex / Formation of annular gap junctions / Gap junction degradation / LDL clearance / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / WNT5A-dependent internalization of FZD4 / clathrin coat / WNT5A-dependent internalization of FZD2, FZD5 and ROR2 / Trafficking of GluR2-containing AMPA receptors / LDL clearance / Retrograde neurotrophin signalling / Retrograde neurotrophin signalling / VLDLR internalisation and degradation / WNT5A-dependent internalization of FZD4 / VLDLR internalisation and degradation / clathrin adaptor complex / cardiac septum development / metalloendopeptidase inhibitor activity / Recycling pathway of L1 / extrinsic component of presynaptic endocytic zone membrane / MHC class II antigen presentation / regulation of vesicle size / postsynaptic endocytic zone / AP-2 adaptor complex / postsynaptic neurotransmitter receptor internalization / Cargo recognition for clathrin-mediated endocytosis / Recycling pathway of L1 / membrane coat / Clathrin-mediated endocytosis / clathrin coat assembly / positive regulation of synaptic vesicle endocytosis / Cargo recognition for clathrin-mediated endocytosis / clathrin adaptor activity / Clathrin-mediated endocytosis / vesicle budding from membrane / clathrin-dependent endocytosis / MHC class II antigen presentation / signal sequence binding / coronary vasculature development / positive regulation of protein localization to membrane / aorta development / ventricular septum development / Neutrophil degranulation / low-density lipoprotein particle receptor binding / clathrin binding / Trafficking of GluR2-containing AMPA receptors / positive regulation of endocytosis / positive regulation of receptor internalization / synaptic vesicle endocytosis / negative regulation of protein localization to plasma membrane / vesicle-mediated transport / phosphatidylinositol binding / protein serine/threonine kinase binding / secretory granule / intracellular protein transport / kidney development / kinase binding / cytoplasmic side of plasma membrane / disordered domain specific binding / synaptic vesicle / heart development / protein-containing complex assembly / cytoplasmic vesicle / defense response to virus / transmembrane transporter binding / postsynapse / protein domain specific binding / innate immune response / intracellular membrane-bounded organelle / lipid binding / synapse / protein kinase binding / GTP binding / protein-containing complex binding / host cell plasma membrane / glutamatergic synapse / cell surface / Golgi apparatus / mitochondrion / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |    Simian immunodeficiency virus Simian immunodeficiency virus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Buffalo CZ / Ren X | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Cell Host Microbe / Year: 2019 Journal: Cell Host Microbe / Year: 2019Title: Structural Basis for Tetherin Antagonism as a Barrier to Zoonotic Lentiviral Transmission. Authors: Cosmo Z Buffalo / Christina M Stürzel / Elena Heusinger / Dorota Kmiec / Frank Kirchhoff / James H Hurley / Xuefeng Ren /   Abstract: Tetherin is a host defense factor that physically prevents virion release from the plasma membrane. The Nef accessory protein of simian immunodeficiency virus (SIV) engages the clathrin adaptor AP-2 ...Tetherin is a host defense factor that physically prevents virion release from the plasma membrane. The Nef accessory protein of simian immunodeficiency virus (SIV) engages the clathrin adaptor AP-2 to downregulate tetherin via its DIWK motif. As human tetherin lacks DIWK, antagonism of tetherin by Nef is a barrier to simian-human transmission of non-human primate lentiviruses. To determine the molecular basis for tetherin counteraction, we reconstituted the AP-2 complex with a simian tetherin and SIV Nef and determined its structure by cryoelectron microscopy (cryo-EM). Nef refolds the first α-helix of the β2 subunit of AP-2 to a β hairpin, creating a binding site for the DIWK sequence. The tetherin binding site in Nef is distinct from those of most other Nef substrates, including MHC class I, CD3, and CD4 but overlaps with the site for the restriction factor SERINC5. This structure explains the dependence of SIVs on tetherin DIWK and consequent barrier to human transmission. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20217.map.gz emd_20217.map.gz | 1.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20217-v30.xml emd-20217-v30.xml emd-20217.xml emd-20217.xml | 21.2 KB 21.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20217_fsc.xml emd_20217_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_20217.png emd_20217.png | 205.6 KB | ||
| Filedesc metadata |  emd-20217.cif.gz emd-20217.cif.gz | 7.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20217 http://ftp.pdbj.org/pub/emdb/structures/EMD-20217 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20217 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20217 | HTTPS FTP |
-Validation report
| Summary document |  emd_20217_validation.pdf.gz emd_20217_validation.pdf.gz | 363.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_20217_full_validation.pdf.gz emd_20217_full_validation.pdf.gz | 362.7 KB | Display | |
| Data in XML |  emd_20217_validation.xml.gz emd_20217_validation.xml.gz | 11 KB | Display | |
| Data in CIF |  emd_20217_validation.cif.gz emd_20217_validation.cif.gz | 14.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20217 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20217 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20217 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20217 | HTTPS FTP |
-Related structure data
| Related structure data |  6owtMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20217.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20217.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.149 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Structure of SIVsmm Nef and SMM tetherin bound to the clathrin ad...
| Entire | Name: Structure of SIVsmm Nef and SMM tetherin bound to the clathrin adaptor AP-2 complex |
|---|---|
| Components |
|
-Supramolecule #1: Structure of SIVsmm Nef and SMM tetherin bound to the clathrin ad...
| Supramolecule | Name: Structure of SIVsmm Nef and SMM tetherin bound to the clathrin adaptor AP-2 complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 203.6 KDa |
-Macromolecule #1: AP-2 complex subunit alpha
| Macromolecule | Name: AP-2 complex subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 104.286391 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPAVSKGEGM RGLAVFISDI RNCKSKEAEI KRINKELANI RSKFKGDKAL DGYSKKKYVC KLLFIFLLGH DIDFGHMEAV NLLSSNRYT EKQIGYLFIS VLVNSNSELI RLINNAIKND LASRNPTFMG LALHCIANVG SREMAEAFAG EIPKILVAGD T MDSVKQSA ...String: MPAVSKGEGM RGLAVFISDI RNCKSKEAEI KRINKELANI RSKFKGDKAL DGYSKKKYVC KLLFIFLLGH DIDFGHMEAV NLLSSNRYT EKQIGYLFIS VLVNSNSELI RLINNAIKND LASRNPTFMG LALHCIANVG SREMAEAFAG EIPKILVAGD T MDSVKQSA ALCLLRLYRT SPDLVPMGDW TSRVVHLLND QHLGVVTAAT SLITTLAQKN PEEFKTSVSL AVSRLSRIVT SA STDLQDY TYYFVPAPWL SVKLLRLLQC YPPPEDPAVR GRLTECLETI LNKAQEPPKS KKVQHSNAKN AVLFEAISLI IHH DSEPNL LVRACNQLGQ FLQHRETNLR YLALESMCTL ASSEFSHEAV KTHIETVINA LKTERDVSVR QRAVDLLYAM CDRS NAQQI VAEMLSYLET ADYSIREEIV LKVAILAEKY AVDYTWYVDT ILNLIRIAGD YVSEEVWYRV IQIVINRDDV QGYAA KTVF EALQAPACHE NLVKVGGYIL GEFGNLIAGD PRSSPLIQFN LLHSKFHLCS VPTRALLLST YIKFVNLFPE VKATIQ DVL RSDSQLKNAD VELQQRAVEY LRLSTVASTD ILATVLEEMP PFPERESSIL AKLKKKKGPS TVTDLEETKR ERSIDVN GG PEPVPASTSA ASTPSPSADL LGLGAVPPAP TGPPPTSGGG LLVDVFSDSA SAVAPLAPGS EDNFARFVCK NNGVLFEN Q LLQIGLKSEF RQNLGRMFIF YGNKTSTQFL NFTPTLICAD DLQTNLNLQT KPVDPTVDGG AQVQQVVNIE CISDFTEAP VLNIQFRYGG TFQNVSVKLP ITLNKFFQPT EMASQDFFQR WKQLSNPQQE VQNIFKAKHP MDTEITKAKI IGFGSALLEE VDPNPANFV GAGIIHTKTT QIGCLLRLEP NLQAQMYRLT LRTSKDTVSQ RLCELLSEQF UniProtKB: AP-2 complex subunit alpha |
-Macromolecule #2: AP-2 complex subunit beta
| Macromolecule | Name: AP-2 complex subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 66.953195 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTDSKYFTTN KKGEIFELKA ELNNEKKEKR KEAVKKVIAA MTVGKDVSSL FPDVVNCMQT DNLELKKLVY LYLMNYAKSQ PDMAIMAVN SFVKDCEDPN PLIRALAVRT MGCIRVDKIT EYLCEPLRKC LKDEDPYVRK TAAVCVAKLH DINAQMVEDQ G FLDSLRDL ...String: MTDSKYFTTN KKGEIFELKA ELNNEKKEKR KEAVKKVIAA MTVGKDVSSL FPDVVNCMQT DNLELKKLVY LYLMNYAKSQ PDMAIMAVN SFVKDCEDPN PLIRALAVRT MGCIRVDKIT EYLCEPLRKC LKDEDPYVRK TAAVCVAKLH DINAQMVEDQ G FLDSLRDL IADSNPMVVA NAVAALSEIS ESHPNSNLLD LNPQNINKLL TALNECTEWG QIFILDCLSN YNPKDDREAQ SI CERVTPR LSHANSAVVL SAVKVLMKFL ELLPKDSDYY NMLLKKLAPP LVTLLSGEPE VQYVALRNIN LIVQKRPEIL KQE IKVFFV KYNDPIYVKL EKLDIMIRLA SQANIAQVLA ELKEYATEVD VDFVRKAVRA IGRCAIKVEQ SAERCVSTLL DLIQ TKVNY VVQEAIVVIR DIFRKYPNKY ESIIATLCEN LDSLDEPDAR AAMIWIVGEY AERIDNADEL LESFLEGFHD ESTQV QLTL LTAIVKLFLK KPSETQELVQ QVLSLATQDS DNPDLRDRGY IYWRLLSTDP VTAKEVVLSE KPLISEETDL IEPTLL DEL ICHIGSLASV YHKPPNAFVE GSHGIHRK UniProtKB: AP-2 complex subunit beta |
-Macromolecule #3: Adaptor protein complex AP-2, mu1
| Macromolecule | Name: Adaptor protein complex AP-2, mu1 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 16.161563 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIGGLFIYNH KGEVLISRVY RDDIGRNAVD AFRVNVIHAR QQVRSPVTNI ARTSFFHVKR SNIWLAAVTK QNVNAAMVFE FLYKMCDVM AAYFGKISEE NIKNNFVLIY ELLDEILDFG YPQNSETGAL KTFITQQGIK SQ UniProtKB: AP-2 complex subunit mu |
-Macromolecule #4: Tetherin,Protein Nef
| Macromolecule | Name: Tetherin,Protein Nef / type: protein_or_peptide / ID: 4 Details: protein chimera of the cytoplasmic tail of sooty mangabey (smm) linked to the N-terminus of SIV smm tetherin Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Simian immunodeficiency virus Simian immunodeficiency virus |
| Molecular weight | Theoretical: 31.772352 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ILYDYSRMPM GDIWKEDGDK RSKGSDEASE GSGMGGVTSK KQSKRRQGLR ERLLQARGET DGYSRSRGEL GKGWNLPSAE GQGYSEEQF MNTPWRNPAR EGEKLKYRQQ NMDDVDDDDD ELVGVAVHPK VPLRAMSYKL AIDMSHFIKE KGGLEGIYYS D RRHRILDI ...String: ILYDYSRMPM GDIWKEDGDK RSKGSDEASE GSGMGGVTSK KQSKRRQGLR ERLLQARGET DGYSRSRGEL GKGWNLPSAE GQGYSEEQF MNTPWRNPAR EGEKLKYRQQ NMDDVDDDDD ELVGVAVHPK VPLRAMSYKL AIDMSHFIKE KGGLEGIYYS D RRHRILDI YLEKEEGIIP DWQNYTSGPG VRYPLFFGWL WKLVPVNVSD EAQEDETHCL VHPAQTSQWD DPWGEVLAWK FD PKLAYTY EAFIRYPEEF GNDSGLSKEE VKRRLTAR UniProtKB: Bone marrow stromal antigen 2, Protein Nef |
-Macromolecule #5: AP-2 complex subunit sigma
| Macromolecule | Name: AP-2 complex subunit sigma / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 17.011662 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIRFILIQNR AGKTRLAKWY MQFDDDEKQK LIEEVHAVVT VRDAKHTNFV EFRNFKIIYR RYAGLYFCIC VDVNDNNLAY LEAIHNFVE VLNEYFHCVS ELDLVFNFYK VYTVVDEMFL AGEIRETSQT KVLKQLLMLQ SLE UniProtKB: AP-2 complex subunit sigma |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.7 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: C-flat-2/1 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.04 kPa / Details: 25mA |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 50.16 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)
























