[English] 日本語
 Yorodumi
Yorodumi- EMDB-19465: Structure of RyR1 reconstituted into lipid liposomes in primed st... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of RyR1 reconstituted into lipid liposomes in primed state in complex with Ca2+, ATP, caffeine and Nb9657. | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Ion channel / Ca2+ / tetramer / TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationATP-gated ion channel activity / terminal cisterna / ryanodine-sensitive calcium-release channel activity / ryanodine receptor complex / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / ossification involved in bone maturation / cellular response to caffeine / skin development / organelle membrane / intracellularly gated calcium channel activity ...ATP-gated ion channel activity / terminal cisterna / ryanodine-sensitive calcium-release channel activity / ryanodine receptor complex / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / ossification involved in bone maturation / cellular response to caffeine / skin development / organelle membrane / intracellularly gated calcium channel activity / smooth endoplasmic reticulum / outflow tract morphogenesis / regulation of ryanodine-sensitive calcium-release channel activity / toxic substance binding / striated muscle contraction / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / voltage-gated calcium channel activity / skeletal muscle fiber development / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / release of sequestered calcium ion into cytosol / sarcoplasmic reticulum membrane / cellular response to calcium ion / muscle contraction / sarcoplasmic reticulum / peptidylprolyl isomerase / peptidyl-prolyl cis-trans isomerase activity / sarcolemma / calcium ion transmembrane transport / Z disc / calcium channel activity / intracellular calcium ion homeostasis / disordered domain specific binding / protein homotetramerization / transmembrane transporter binding / calmodulin binding / intracellular membrane-bounded organelle / calcium ion binding / ATP binding / identical protein binding / membrane Similarity search - Function | ||||||||||||
| Biological species |   | ||||||||||||
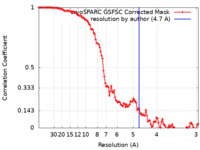
| Method | single particle reconstruction / cryo EM / Resolution: 4.7 Å | ||||||||||||
 Authors Authors | Li C / Efremov RG | ||||||||||||
| Funding support |  Belgium, European Union, 3 items Belgium, European Union, 3 items
| ||||||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2024 Journal: J Biol Chem / Year: 2024Title: Rapid small-scale nanobody-assisted purification of ryanodine receptors for cryo-EM. Authors: Chenyao Li / Katrien Willegems / Tomasz Uchański / Els Pardon / Jan Steyaert / Rouslan G Efremov /  Abstract: Ryanodine receptors (RyRs) are large Ca release channels residing in the endoplasmic or sarcoplasmic reticulum membrane. Three isoforms of RyRs have been identified in mammals, the disfunction of ...Ryanodine receptors (RyRs) are large Ca release channels residing in the endoplasmic or sarcoplasmic reticulum membrane. Three isoforms of RyRs have been identified in mammals, the disfunction of which has been associated with a series of life-threatening diseases. The need for large amounts of native tissue or eukaryotic cell cultures limits advances in structural studies of RyRs. Here, we report a method that utilizes nanobodies to purify RyRs from only 5 mg of total protein. The purification process, from isolated membranes to cryo-EM grade protein, is achieved within 4 h on the bench, yielding protein usable for cryo-EM analysis. This is demonstrated by solving the structures of rabbit RyR1, solubilized in detergent, reconstituted into lipid nanodiscs or liposomes, and bovine RyR2 reconstituted in nanodisc, and mouse RyR2 in detergent. The reported method facilitates structural studies of RyRs directed toward drug development and is useful in cases where the amount of starting material is limited. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19465.map.gz emd_19465.map.gz | 131.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19465-v30.xml emd-19465-v30.xml emd-19465.xml emd-19465.xml | 20.6 KB 20.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19465_fsc.xml emd_19465_fsc.xml | 11.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_19465.png emd_19465.png | 86 KB | ||
| Filedesc metadata |  emd-19465.cif.gz emd-19465.cif.gz | 9.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19465 http://ftp.pdbj.org/pub/emdb/structures/EMD-19465 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19465 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19465 | HTTPS FTP |
-Related structure data
| Related structure data |  8rruMC  8rrsC  8rrtC  8rrvC  8rrwC  8rrxC  8rs0C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19465.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19465.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.5 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : Ryanodine receptor 1 in complex with nanobody and FKBP12
+Supramolecule #1: Ryanodine receptor 1 in complex with nanobody and FKBP12
+Supramolecule #2: Ryanodine receptor 1 in complex with FKBP12
+Supramolecule #3: Nanobody 9657
+Macromolecule #1: Peptidyl-prolyl cis-trans isomerase FKBP1B
+Macromolecule #2: Ryanodine receptor 1
+Macromolecule #3: Nanobody 9657
+Macromolecule #4: ZINC ION
+Macromolecule #5: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #6: CAFFEINE
+Macromolecule #7: CALCIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)