[English] 日本語
 Yorodumi
Yorodumi- EMDB-19463: Structure of mouse RyR2 solubilised in detergent in open state in... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of mouse RyR2 solubilised in detergent in open state in complex with Ca2+, ATP, caffeine and Nb9657. | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Ion channel / Ca2+ / tetramer / TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationestablishment of protein localization to endoplasmic reticulum / type B pancreatic cell apoptotic process / Purkinje myocyte to ventricular cardiac muscle cell signaling / regulation of atrial cardiac muscle cell action potential / left ventricular cardiac muscle tissue morphogenesis / suramin binding / regulation of AV node cell action potential / regulation of SA node cell action potential / Stimuli-sensing channels / regulation of ventricular cardiac muscle cell action potential ...establishment of protein localization to endoplasmic reticulum / type B pancreatic cell apoptotic process / Purkinje myocyte to ventricular cardiac muscle cell signaling / regulation of atrial cardiac muscle cell action potential / left ventricular cardiac muscle tissue morphogenesis / suramin binding / regulation of AV node cell action potential / regulation of SA node cell action potential / Stimuli-sensing channels / regulation of ventricular cardiac muscle cell action potential / ventricular cardiac muscle cell action potential / : / embryonic heart tube morphogenesis / Ion homeostasis / cardiac muscle hypertrophy / calcium ion transport into cytosol / ryanodine-sensitive calcium-release channel activity / response to caffeine / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / response to redox state / cellular response to caffeine / calcium ion transmembrane import into cytosol / response to muscle activity / protein kinase A catalytic subunit binding / protein kinase A regulatory subunit binding / positive regulation of the force of heart contraction / intracellularly gated calcium channel activity / smooth endoplasmic reticulum / detection of calcium ion / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / positive regulation of heart rate / response to muscle stretch / cellular response to epinephrine stimulus / calcium channel complex / sarcoplasmic reticulum membrane / regulation of heart rate / sarcomere / sarcoplasmic reticulum / establishment of localization in cell / calcium-mediated signaling / calcium ion transmembrane transport / Z disc / calcium channel activity / intracellular calcium ion homeostasis / calcium ion transport / response to hypoxia / calmodulin binding / calcium ion binding / protein kinase binding / enzyme binding / protein-containing complex / identical protein binding / membrane Similarity search - Function | ||||||||||||
| Biological species |   | ||||||||||||
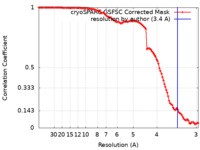
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | ||||||||||||
 Authors Authors | Li C / Efremov RG | ||||||||||||
| Funding support |  Belgium, European Union, 3 items Belgium, European Union, 3 items
| ||||||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2024 Journal: J Biol Chem / Year: 2024Title: Rapid small-scale nanobody-assisted purification of ryanodine receptors for cryo-EM. Authors: Chenyao Li / Katrien Willegems / Tomasz Uchański / Els Pardon / Jan Steyaert / Rouslan G Efremov /  Abstract: Ryanodine receptors (RyRs) are large Ca release channels residing in the endoplasmic or sarcoplasmic reticulum membrane. Three isoforms of RyRs have been identified in mammals, the disfunction of ...Ryanodine receptors (RyRs) are large Ca release channels residing in the endoplasmic or sarcoplasmic reticulum membrane. Three isoforms of RyRs have been identified in mammals, the disfunction of which has been associated with a series of life-threatening diseases. The need for large amounts of native tissue or eukaryotic cell cultures limits advances in structural studies of RyRs. Here, we report a method that utilizes nanobodies to purify RyRs from only 5 mg of total protein. The purification process, from isolated membranes to cryo-EM grade protein, is achieved within 4 h on the bench, yielding protein usable for cryo-EM analysis. This is demonstrated by solving the structures of rabbit RyR1, solubilized in detergent, reconstituted into lipid nanodiscs or liposomes, and bovine RyR2 reconstituted in nanodisc, and mouse RyR2 in detergent. The reported method facilitates structural studies of RyRs directed toward drug development and is useful in cases where the amount of starting material is limited. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19463.map.gz emd_19463.map.gz | 132 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19463-v30.xml emd-19463-v30.xml emd-19463.xml emd-19463.xml | 19.2 KB 19.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19463_fsc.xml emd_19463_fsc.xml | 11 KB | Display |  FSC data file FSC data file |
| Images |  emd_19463.png emd_19463.png | 97.1 KB | ||
| Filedesc metadata |  emd-19463.cif.gz emd-19463.cif.gz | 9.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19463 http://ftp.pdbj.org/pub/emdb/structures/EMD-19463 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19463 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19463 | HTTPS FTP |
-Related structure data
| Related structure data |  8rrsMC  8rrtC  8rruC  8rrvC  8rrwC  8rrxC  8rs0C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19463.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19463.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.46 Å | ||||||||||||||||||||||||||||||||||||
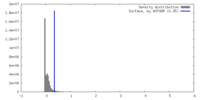
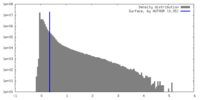
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Mouse ryanodine receptor 2 complex with nanobody
| Entire | Name: Mouse ryanodine receptor 2 complex with nanobody |
|---|---|
| Components |
|
-Supramolecule #1: Mouse ryanodine receptor 2 complex with nanobody
| Supramolecule | Name: Mouse ryanodine receptor 2 complex with nanobody / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|
-Supramolecule #2: Mouse ryanodine receptor 2
| Supramolecule | Name: Mouse ryanodine receptor 2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: Nanobody 9657
| Supramolecule | Name: Nanobody 9657 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Ryanodine receptor 2
| Macromolecule | Name: Ryanodine receptor 2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 565.536 KDa |
| Sequence | String: MADAGEGEDE IQFLRTDDEV VLQCTATIHK EQQKLCLAAE GFGNRLCFLE STSNSKNVPP DLSICTFVLE QSLSVRALQE MLANTVEKS EGQVDVEKWK FMMKTAQGGG HRTLLYGHAI LLRHSYSGMY LCCLSTSRSS TDKLAFDVGL QEDTTGEACW W TIHPASKQ ...String: MADAGEGEDE IQFLRTDDEV VLQCTATIHK EQQKLCLAAE GFGNRLCFLE STSNSKNVPP DLSICTFVLE QSLSVRALQE MLANTVEKS EGQVDVEKWK FMMKTAQGGG HRTLLYGHAI LLRHSYSGMY LCCLSTSRSS TDKLAFDVGL QEDTTGEACW W TIHPASKQ RSEGEKVRVG DDLILVSVSS ERYLHLSYGN SSWHVDAAFQ QTLWSVAPIS SGSEAAQGYL IGGDVLRLLH GH MDECLTV PSGEHGEEQR RTVHYEGGAV SVHARSLWRL ETLRVAWSGS HIRWGQPFRL RHVTTGKYLS LMEDKNLLLM DKE KADVKS TAFAFRSSKE KLDVGVRKEV DGMGTSEIKY GDSICYIQHV DTGLWLTYQA VDVKSARMGS IQRKAIMHHE GHMD DGLNL SRSQHEESRT ARVIRSTVFL FNRFIRGLDA LSKKVKLPTI DLPIESVSLS LQDLIGYFHP PDEHLEHEDK QNRLR ALKN RQNLFQEEGM INLVLECIDR LHVYSSAAHF ADVAGREAGE SWKSILNSLY ELLAALIRGN RKNCAQFSGS LDWLIS RLE RLEASSGILE VLHCVLVESP EALNIIKEGH IKSIISLLDK HGRNHKVLDV LCSLCVCHGV AVRSNQHLIC DNLLPGR DL LLQTRLVNHV SSMRPNIFLG VSEGSAQYKK WYYELMVDHT EPFVTAEATH LRVGWASTEG YSPYPGGGEE WGGNGVGD D LFSYGFDGLH LWSGCIARTV SSPNQHLLRT DDVISCCLDL SAPSISFRIN GQPVQGMFEN FNIDGLFFPV VSFSAGIKV RFLLGGRHGE FKFLPPPGYA ACYEAVLPKE KLKVEHSREY KQERTYTRDL LGPTVSLTQA AFTPVPVDTS QIVLPPHLER IRERLAENI HELWVMNKIE LGWQYGPVRD DNKRQHPCLV EFCKLPEQER NYNLQMSLET LKTLLALGCH VGIADEHAEE K VKKMKLPK NYQLTSGYKP APMDLSFIKL TPSQEAMVDK LAENAHNVWA RDRIRQGWTY GIQQDVKNRR NPRLVPYTLL DD RTKKSNK DSLREAVRTL LGYGYHLEAP DQDHASRAEV CSGTGERFRI FRAEKTYAVK AGRWYFEFEA VTAGDMRVGW SRP GCQPDL ELGSDDRAFA FDGFKAQRWH QGNEHYGRSW QAGDVVGCMV DMNEHTMMFT LNGEILLDDS GSELAFKDFD VGDG FIPVC SLGVAQVGRM NFGKDVSTLK YFTICGLQEG YEPFAVNTNR DITMWLSKRL PQFLQVPSNH EHIEVTRIDG TIDSS PCLK VTQKSFGSQN NNTDIMFYRL SMPIECAEVF SKSVAGGLPG AGFYGPKNDL EDFDVDSDFE VLMKTAHGHL VPDRID KDK ETPKPEFNNH KDYAQEKPSR LKQRFLLRRT KPDYSTGHSA RLTEDVLADD RDDYEYLMQT STYYYSVRIF PGQEPAN VW VGWITSDFHQ YDTGFDLDRV RTVTVTLGDE KGKVHESIKR SNCYMVCAGE SMSPGQGRNN SNGLEIGCVV DAASGLLT F IANGKELSTY YQVEPSTKLF PAVFAQATSP NVFQFELGRI KNVMPLSAGL FKSEHKNPVP QCPPRLHVQF LSHVLWSRM PNQFLKVDVS RISERQGWLV QCLDPLQFMS LHIPEENRSV DILELTEQEE LLQFHYHTLR LYSAVCALGN HRVAHALCSH VDEPQLLYA IENKYMPGLL RAGYYDLLID IHLSSYATAR LMMNNEFIVP MTEETKSITL FPDENKKHGL PGIGLSTSLR P RMRFSSPS FVSISNDCYQ YSPEFPLDIL KAKTIQMLTE AVKEGSLHAR DPVGGTTEFL FVPLIKLFYT LLIMGIFHNE DL KHILQLI EPSVFKEAAV PEEEGGTPEK EISIEDAKLE GEEEAKGGKR PKEGLLQMKL PEPVKLQMCL LLQYLCDCQV RHR IEAIVA FSDDFVAKLQ DNQRFRYNEV MQALNMSAAL TARKTREFRS PPQEQINMLL NFKDDKSECP CPEEIRDQLL DFHE DLMTH CGIELDEDGS LDGSNDLTIR GRLLSLVEKV TYLKKKQAEK PVASDSRKCS SLQQLISETM VRWAQESVIE DPELV RAMF VLLHRQYDGI GGLVRALPKT YTINGVSVED TINLLASLGQ IRSLLSVRMG KEEEKLMIRG LGDIMNNKVF YQHPNL MRA LGMHETVMEV MVNVLGGGES KEITFPKMVA NCCRFLCYFC RISRQNQKAM FDHLSYLLEN SSVGLASPAM RGSTPLD VA AASVMDNNEL ALALREPDLE KVVRYLAGCG LQSCQMLVSK GYPDIGWNPV EGERYLDFLR FAVFCNGESV EENANVVV R LLIRRPECFG PALRGEGGNG LLAAMEEAIK IAEDPSRDGP SPTSGSSKTL DIEEEEDDTI HMGNAIMTFY AALIDLLGR CAPEMHLIHA GKGEAIRIRS ILRSLIPLGD LVGVISIAFQ MPTIAKDGKV VEPDMSAGFC PDHKAAMVLF LDRVYGIEVQ DFLLHLLEV GFLPDLRAAA SLDTAALSAT DMALALNRYL CTAVLPLLTR CAPLFAGTEH HASLIDSLLH TVYRLSKGCS L TKAQRDSI EVCLLSICGQ LRPSMMQHLL RRLVFDVPLL NEHAKMPLKL LTNHYERCWK YYCLPGGWGN FGAASEEELH LS RKLFWGI FDALSQKKYE QELFKLALPC LSAVAGALPP DYMESNYVSM MEKQSSMDSE GNFNPQPVDT SNITIPEKLE YFI NKYAEH SHDKWSMDKL ANGWIYGEIY SDSSKIQPLM KPYKLLSEKE KEIYRWPIKE SLKTMLAWGW RIERTREGDS MALY NRTRR ISQTSQVSID AAHGYSPRAI DMSNVTLSRD LHAMAEMMAE NYHNIWAKKK KLELESKGGG NHPLLVPYDT LTAKE KAKD REKAQDIFKF LQISGYVVSR GFKDLDLDTP SIEKRFAYSF LQQLIRYVDE AHQYILEFDG GSRSKGEHFP YEQEIK FFA KVVLPLIDQY FKNHRLYFLS AASRPLCTGG HASNKEKEMV TSLFCKLGVL VRHRISLFGN DATSIVNCLH ILGQTLD AR TVMKTGLDSV KSALRAFLDN AAEDLEKTME NLKQGQFTHT RSQPKGVTQI INYTTVALLP MLSSLFEHIG QHQFGEDL I LEDVQVSCYR ILTSLYALGT SKSIYVERQR SALGECLAAF AGAFPIAFLE THLDKHNVYS IYNTRSSRER AALSLPANV EDVCPNIPSL EKLMTEIIEL AESGIRYTQM PYMMEVVLPM LCSYMSRWWE HGPENHPERA EMCCTALNSE HMNTLLGNIL KIIYNNLGI DEGAWMKRLA VFSQPIINKV KPQLLKTHFL PLMEKLKKKA AMVVSEEDHL KAEARGDMSE AELLILDEFT T LARDLYAF YPLLIRFVDY NRAKWLKEPN PEAEELFRMV AEVFIYWSKS HNFKREEQNF VVQNEINNMS FLITDTKSKM SK AAISDQE RKKMKRKGDR YSMQTSLIVA ALKRLLPIGL NICAPGDQEL IALAKNRFSL KDTEEEVRDI IRSNIHLQGK LED PAIRWQ MALYKDLPNR TEDPSDPERT VERVLGIANV LFHLEQKSKY TGRGYFSLVE HPQRSKKAVW HKLLSKQRKR AVVA CFRMA PLYNLPRHRA VNLFLQGYEK SWIETEEHYF EDKLIEDLAK PGAELPEEDE AMKRVDPLHQ LILLFSRTAL TEKCK LEED FLYMAYADIM AKSCHDEEDD DGEEEVKSFE EKEMEKQKLL YQQARLHDRG AAEMVLQTIS ASKGETGPMV AATLKL GIA ILNGGNSTVQ QKMLDYLKEK KDVGFFQSLA GLMQSCSVLD LNAFERQNKA EGLGMVTEEG SGEKVLQDDE FTCDLFR FL QLLCEGHNSD FQNYLRTQTG NNTTVNIIIS TVDYLLRVQE SISDFYWYYS GKDIIDEQGQ RNFSKAIQVA KQVFNTLT E YIQGPCTGNQ QSLAHSRLWD AVVGFLHVFA HMQMKLSQDS SQIELLKELM DLQKDMVVML LSMLEGNVVN GTIGKQMVD MLVESSNNVE MILKFFDMFL KLKDLTSSDT FKEYDPDGKG VISKRDFHKA MESHKHYTQS ETEFLLSCAE TDENETLDYE EFVKRFHEP AKDIGFNVAV LLTNLSEHMP NDTRLQTFLE LAESVLNYFQ PFLGRIEIMG SAKRIERVYF EISESSRTQW E KPQVKESK RQFIFDVVNE GGEKEKMELF VNFCEDTIFE MQLAAQISES DLNERLANKE ESEKERPEEQ APRMGFFSLL TI QSALFAL RYNVLTLVRM LSLKSLKKQM KRMKKMTVKD MVLAFFSSYW SVFVTLLHFV ASVCRGFFRI VSSLLLGGSL VEG AKKIKV AELLANMPDP TQDEVRGDEE EGERKPLESA LPSEDLTDLK ELTEESDLLS DIFGLDLKRE GGQYKLIPHN PNAG LSDLM TNPVPVPEVQ EKFQEQKAKE EKEEKEETKS EPEKAEGEDG EKEEKAKDEK SKQKLRQLHT HRYGEPEVPE SAFWK KIIA YQQKLLNYFA RNFYNMRMLA LFVAFAINFI LLFYKVSTSS VVEGKELPTR TSSDTAKVTN SLDSSPHRII AVHYVL EES SGYMEPTLRI LAILHTIISF FCIIGYYCLK VPLVIFKREK EVARKLEFDG LYITEQPSED DIKGQWDRLV INTQSFP NN YWDKFVKRKV MDKYGEFYGR DRISELLGMD KAALDFSDAR EKKKPKKDSS LSAVLNSIDV KYQMWKLGVV FTDNSFLY L AWYMTMSVLG HYNNFFFAAH LLDIAMGFKT LRTILSSVTH NGKQLVLTVG LLAVVVYLYT VVAFNFFRKF YNKSEDGDT PDMKCDDMLT CYMFHMYVGV RAGGGIGDEI EDPAGDEYEI YRIIFDITFF FFVIVILLAI IQGLIIDAFG ELRDQQEQVK EDMETKCFI CGIGNDYFDT VPHGFETHTL QEHNLANYLF FLMYLINKDE TEHTGQESYV WKMYQERCWE FFPAGDCFRK Q YEDQLN UniProtKB: Ryanodine receptor 2 |
-Macromolecule #2: Nanobody 9657
| Macromolecule | Name: Nanobody 9657 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.125495 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LMQAGGSLRL SCTASGSIFS INSMGWYRQA PGKQRELVAT ITSGNSINYA DSVKGRFTIS RDNAKNTVYL QMNSLKPED TAVYYCNADR VPNGYNPWGT PNDEYDYWGQ GTQVTVSSHH HHHHEPEA |
-Macromolecule #3: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 4 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #4: CAFFEINE
| Macromolecule | Name: CAFFEINE / type: ligand / ID: 4 / Number of copies: 4 / Formula: CFF |
|---|---|
| Molecular weight | Theoretical: 194.191 Da |
| Chemical component information | 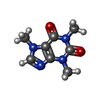 ChemComp-CFF: |
-Macromolecule #5: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 5 / Number of copies: 4 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #6: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 6 / Number of copies: 4 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
 Movie
Movie Controller
Controller





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)