[English] 日本語
 Yorodumi
Yorodumi- EMDB-13065: Cryo-EM structure of ALC1/CHD1L bound to a PARylated nucleosome -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13065 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of ALC1/CHD1L bound to a PARylated nucleosome | |||||||||||||||
 Map data Map data | Main map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | ALC1 / CHD1L / chromatin remodeler / DNA damage response / nucleosome / poly(ADP-ribose) / DNA BINDING PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpoly-ADP-D-ribose modification-dependent protein binding / ATP-dependent chromatin remodeler activity / histone reader activity / site of DNA damage / nucleosome binding / DNA helicase activity / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / Dual Incision in GG-NER / Formation of Incision Complex in GG-NER / structural constituent of chromatin ...poly-ADP-D-ribose modification-dependent protein binding / ATP-dependent chromatin remodeler activity / histone reader activity / site of DNA damage / nucleosome binding / DNA helicase activity / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / Dual Incision in GG-NER / Formation of Incision Complex in GG-NER / structural constituent of chromatin / heterochromatin formation / nucleosome / nucleosome assembly / site of double-strand break / chromatin remodeling / protein heterodimerization activity / nucleotide binding / DNA repair / DNA damage response / ATP hydrolysis activity / DNA binding / nucleoplasm / ATP binding / nucleus Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||||||||
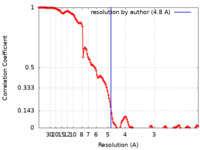
| Method | single particle reconstruction / cryo EM / Resolution: 4.8 Å | |||||||||||||||
 Authors Authors | Bacic L / Gaullier G | |||||||||||||||
| Funding support |  Sweden, 4 items Sweden, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Elife / Year: 2021 Journal: Elife / Year: 2021Title: Structure and dynamics of the chromatin remodeler ALC1 bound to a PARylated nucleosome. Authors: Luka Bacic / Guillaume Gaullier / Anton Sabantsev / Laura C Lehmann / Klaus Brackmann / Despoina Dimakou / Mario Halic / Graeme Hewitt / Simon J Boulton / Sebastian Deindl /    Abstract: The chromatin remodeler ALC1 is recruited to and activated by DNA damage-induced poly(ADP-ribose) (PAR) chains deposited by PARP1/PARP2/HPF1 upon detection of DNA lesions. ALC1 has emerged as a ...The chromatin remodeler ALC1 is recruited to and activated by DNA damage-induced poly(ADP-ribose) (PAR) chains deposited by PARP1/PARP2/HPF1 upon detection of DNA lesions. ALC1 has emerged as a candidate drug target for cancer therapy as its loss confers synthetic lethality in homologous recombination-deficient cells. However, structure-based drug design and molecular analysis of ALC1 have been hindered by the requirement for PARylation and the highly heterogeneous nature of this post-translational modification. Here, we reconstituted an ALC1 and PARylated nucleosome complex modified in vitro using PARP2 and HPF1. This complex was amenable to cryo-EM structure determination without cross-linking, which enabled visualization of several intermediate states of ALC1 from the recognition of the PARylated nucleosome to the tight binding and activation of the remodeler. Functional biochemical assays with PARylated nucleosomes highlight the importance of nucleosomal epitopes for productive remodeling and suggest that ALC1 preferentially slides nucleosomes away from DNA breaks. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13065.map.gz emd_13065.map.gz | 121.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13065-v30.xml emd-13065-v30.xml emd-13065.xml emd-13065.xml | 27.3 KB 27.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13065_fsc.xml emd_13065_fsc.xml | 13.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_13065.png emd_13065.png | 79.2 KB | ||
| Masks |  emd_13065_msk_1.map emd_13065_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13065.cif.gz emd-13065.cif.gz | 7.8 KB | ||
| Others |  emd_13065_half_map_1.map.gz emd_13065_half_map_1.map.gz emd_13065_half_map_2.map.gz emd_13065_half_map_2.map.gz | 226.8 MB 226.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13065 http://ftp.pdbj.org/pub/emdb/structures/EMD-13065 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13065 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13065 | HTTPS FTP |
-Related structure data
| Related structure data |  7otqMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13065.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13065.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0752 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
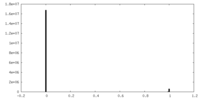
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13065_msk_1.map emd_13065_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
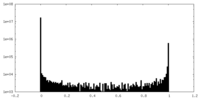
| Density Histograms |
-Half map: Half map 2
| File | emd_13065_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
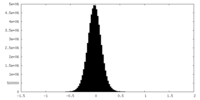
| Density Histograms |
-Half map: Half map 1
| File | emd_13065_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
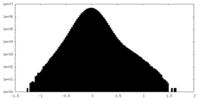
| Density Histograms |
- Sample components
Sample components
-Entire : ALC1/CHD1L bound to a PARylated nucleosome
| Entire | Name: ALC1/CHD1L bound to a PARylated nucleosome |
|---|---|
| Components |
|
-Supramolecule #1: ALC1/CHD1L bound to a PARylated nucleosome
| Supramolecule | Name: ALC1/CHD1L bound to a PARylated nucleosome / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: The nucleosome was PARylated by PARP2 and HPF1 before addition of ALC1. |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 305 KDa |
-Macromolecule #1: Chromodomain-helicase-DNA-binding protein 1-like
| Macromolecule | Name: Chromodomain-helicase-DNA-binding protein 1-like / type: protein_or_peptide / ID: 1 / Details: C-terminal 6-His tag. / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA helicase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 98.906766 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFLLRLHTEG RAEAARVQEQ DLRQWGLTGI HLRSYQLEGV NWLAQRFHCQ NGCILGDEMG LGKTCQTIAL FIYLAGRLND EGPFLILCP LSVLSNWKEE MQRFAPGLSC VTYAGDKEER ACLQQDLKQE SRFHVLLTTY EICLKDASFL KSFPWSVLVV D EAHRLKNQ ...String: MFLLRLHTEG RAEAARVQEQ DLRQWGLTGI HLRSYQLEGV NWLAQRFHCQ NGCILGDEMG LGKTCQTIAL FIYLAGRLND EGPFLILCP LSVLSNWKEE MQRFAPGLSC VTYAGDKEER ACLQQDLKQE SRFHVLLTTY EICLKDASFL KSFPWSVLVV D EAHRLKNQ SSLLHKTLSE FSVVFSLLLT GTPIQNSLQE LYSLLSFVEP DLFSKEEVGD FIQRYQDIEK ESESASELHK LL QPFLLRR VKAEVATELP KKTEVVIYHG MSALQKKYYK AILMKDLDAF ENETAKKVKL QNILSQLRKC VDHPYLFDGV EPE PFEVGD HLTEASGKLH LLDKLLAFLY SGGHRVLLFS QMTQMLDILQ DYMDYRGYSY ERVDGSVRGE ERHLAIKNFG QQPI FVFLL STRAGGVGMN LTAADTVIFV DSDFNPQNDL QAAARAHRIG QNKSVKVIRL IGRDTVEEIV YRKAASKLQL TNMII EGGH FTLGAQKPAA DADLQLSEIL KFGLDKLLAS EGSTMDEIDL ESILGETKDG QWVSDALPAA EGGSRDQEEG KNHMYL FEG KDYSKEPSKE DRKSFEQLVN LQKTLLEKAS QEGRSLRNKG SVLIPGLVEG STKRKRVLSP EELEDRQKKR QEAAAKR RR LIEEKKRQKE EAEHKKKMAW WESNNYQSFC LPSEESEPED LENGEESSAE LDYQDPDATS LKYVSGDVTH PQAGAEDA L IVHCVDDSGH WGRGGLFTAL EKRSAEPRKI YELAGKMKDL SLGGVLLFPV DDKESRNKGQ DLLALIVAQH RDRSNVLSG IKMAALEEGL KKIFLAAKKK KASVHLPRIG HATKGFNWYG TERLIRKHLA ARGIPTYIYY FPRSKAHHHH HH UniProtKB: Chromodomain-helicase-DNA-binding protein 1-like |
-Macromolecule #2: Histone H3.2
| Macromolecule | Name: Histone H3.2 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 15.403062 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MARTKQTARK STGGKAPRKQ LATKAARKSA PATGGVKKPH RYRPGTVALR EIRRYQKSTE LLIRKLPFQR LVREIAQDFK TDLRFQSSA VMALQEASEA YLVALFEDTN LAAIHAKRVT IMPKDIQLAR RIRGERA UniProtKB: Histone H3.2 |
-Macromolecule #3: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 11.394426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Macromolecule #4: Histone H2A type 1
| Macromolecule | Name: Histone H2A type 1 / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 14.109436 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKQGGK TRAKAKTRSS RAGLQFPVGR VHRLLRKGNY AERVGAGAPV YLAAVLEYLT AEILELAGNA ARDNKKTRII PRHLQLAVR NDEELNKLLG RVTIAQGGVL PNIQSVLLPK KTESSKSAKS K UniProtKB: Histone H2A type 1 |
-Macromolecule #5: Histone H2B 1.1
| Macromolecule | Name: Histone H2B 1.1 / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 13.655948 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAKSAPAPKK GSKKAVTKTQ KKDGKKRRKT RKESYAIYVY KVLKQVHPDT GISSKAMSIM NSFVNDVFER IAGEASRLAH YNKRSTITS REIQTAVRLL LPGELAKHAV SEGTKAVTKY TSAK UniProtKB: Histone H2B 1.1 |
-Macromolecule #6: DNA (149-MER) Widom 601 sequence
| Macromolecule | Name: DNA (149-MER) Widom 601 sequence / type: dna / ID: 6 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 49.175336 KDa |
| Sequence | String: (DT)(DC)(DT)(DA)(DG)(DG)(DT)(DG)(DA)(DC) (DC)(DA)(DT)(DC)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG) (DA)(DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA) (DT)(DT)(DG)(DG)(DT)(DC) ...String: (DT)(DC)(DT)(DA)(DG)(DG)(DT)(DG)(DA)(DC) (DC)(DA)(DT)(DC)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG) (DA)(DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA) (DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT) (DA)(DG)(DA)(DC)(DA)(DG)(DC)(DT)(DC)(DT) (DA)(DG) (DC)(DA)(DC)(DC)(DG)(DC)(DT) (DT)(DA)(DA)(DA)(DC)(DG)(DC)(DA)(DC)(DG) (DT)(DA)(DC) (DG)(DC)(DG)(DC)(DT)(DG) (DT)(DC)(DC)(DC)(DC)(DC)(DG)(DC)(DG)(DT) (DT)(DT)(DT)(DA) (DA)(DC)(DC)(DG)(DC) (DC)(DA)(DA)(DG)(DG)(DG)(DG)(DA)(DT)(DT) (DA)(DC)(DT)(DC)(DC) (DC)(DT)(DA)(DG) (DT)(DC)(DT)(DC)(DC)(DA)(DG)(DG)(DC)(DA) (DC)(DG)(DT)(DG)(DT)(DC) (DA)(DG)(DA) (DT)(DA)(DT)(DA)(DT)(DA)(DC)(DA)(DT)(DC) (DG)(DA)(DT)(DA)(DG)(DG)(DC) |
-Macromolecule #7: DNA (149-MER) Widom 601 sequence
| Macromolecule | Name: DNA (149-MER) Widom 601 sequence / type: dna / ID: 7 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 49.606586 KDa |
| Sequence | String: (DG)(DC)(DC)(DT)(DA)(DT)(DC)(DG)(DA)(DT) (DG)(DT)(DA)(DT)(DA)(DT)(DA)(DT)(DC)(DT) (DG)(DA)(DC)(DA)(DC)(DG)(DT)(DG)(DC) (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DC)(DT)(DA) (DG) (DG)(DG)(DA)(DG)(DT)(DA) ...String: (DG)(DC)(DC)(DT)(DA)(DT)(DC)(DG)(DA)(DT) (DG)(DT)(DA)(DT)(DA)(DT)(DA)(DT)(DC)(DT) (DG)(DA)(DC)(DA)(DC)(DG)(DT)(DG)(DC) (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DC)(DT)(DA) (DG) (DG)(DG)(DA)(DG)(DT)(DA)(DA)(DT) (DC)(DC)(DC)(DC)(DT)(DT)(DG)(DG)(DC)(DG) (DG)(DT) (DT)(DA)(DA)(DA)(DA)(DC)(DG) (DC)(DG)(DG)(DG)(DG)(DG)(DA)(DC)(DA)(DG) (DC)(DG)(DC) (DG)(DT)(DA)(DC)(DG)(DT) (DG)(DC)(DG)(DT)(DT)(DT)(DA)(DA)(DG)(DC) (DG)(DG)(DT)(DG) (DC)(DT)(DA)(DG)(DA) (DG)(DC)(DT)(DG)(DT)(DC)(DT)(DA)(DC)(DG) (DA)(DC)(DC)(DA)(DA) (DT)(DT)(DG)(DA) (DG)(DC)(DG)(DG)(DC)(DC)(DT)(DC)(DG)(DG) (DC)(DA)(DC)(DC)(DG)(DG) (DG)(DA)(DT) (DT)(DC)(DT)(DG)(DA)(DT)(DG)(DG)(DT)(DC) (DA)(DC)(DC)(DT)(DA)(DG)(DA) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.04 kPa / Details: Current 20 mA. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: 3 uL were applied on grid and immediately blotted for 2.5 s at blot force 0.. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 26747 / Average exposure time: 2.2 sec. / Average electron dose: 45.0 e/Å2 / Details: Total dose was fractionated over 40 movie frames. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: Real-space CC | ||||||
| Output model |  PDB-7otq: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)