+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6880 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Snf2-nucleosome complex in ADP state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | complex / nucleosome / chromatin remodeling / gene regulation / STRUCTURAL PROTEIN-HYDROLASE-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of cell adhesion involved in single-species biofilm formation / positive regulation of mating type switching / positive regulation of invasive growth in response to glucose limitation / aggrephagy / DNA strand invasion / rDNA binding / nucleosome array spacer activity / : / ATP-dependent chromatin remodeler activity / SWI/SNF complex ...positive regulation of cell adhesion involved in single-species biofilm formation / positive regulation of mating type switching / positive regulation of invasive growth in response to glucose limitation / aggrephagy / DNA strand invasion / rDNA binding / nucleosome array spacer activity / : / ATP-dependent chromatin remodeler activity / SWI/SNF complex / nucleosomal DNA binding / histone H4 reader activity / histone reader activity / transcription initiation-coupled chromatin remodeling / cellular response to amino acid starvation / helicase activity / DNA-templated DNA replication / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / structural constituent of chromatin / heterochromatin formation / nucleosome / double-strand break repair / nucleosome assembly / RNA polymerase II-specific DNA-binding transcription factor binding / chromatin remodeling / protein heterodimerization activity / hydrolase activity / chromatin binding / regulation of transcription by RNA polymerase II / chromatin / positive regulation of transcription by RNA polymerase II / DNA binding / nucleoplasm / ATP binding / nucleus Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.62 Å | |||||||||
 Authors Authors | Li M / Xia X | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Mechanism of DNA translocation underlying chromatin remodelling by Snf2. Authors: Meijing Li / Xian Xia / Yuanyuan Tian / Qi Jia / Xiaoyu Liu / Ying Lu / Ming Li / Xueming Li / Zhucheng Chen /  Abstract: Chromatin remodellers include diverse enzymes with distinct biological functions, but nucleosome-sliding activity appears to be a common theme. Among the remodelling enzymes, Snf2 serves as the ...Chromatin remodellers include diverse enzymes with distinct biological functions, but nucleosome-sliding activity appears to be a common theme. Among the remodelling enzymes, Snf2 serves as the prototype to study the action of this protein family. Snf2 and related enzymes share two conserved RecA-like lobes, which by themselves are able to couple ATP hydrolysis to chromatin remodelling. The mechanism by which these enzymes couple ATP hydrolysis to translocate the nucleosome along the DNA remains unclear. Here we report the structures of Saccharomyces cerevisiae Snf2 bound to the nucleosome in the presence of ADP and ADP-BeF. Snf2 in the ADP-bound state adopts an open conformation similar to that in the apo state, and induces a one-base-pair DNA bulge at superhelix location 2 (SHL2), with the tracking strand showing greater distortion than the guide strand. The DNA distortion propagates to the proximal end, leading to staggered translocation of the two strands. The binding of ADP-BeF triggers a closed conformation of the enzyme, resetting the nucleosome to a relaxed state. Snf2 shows altered interactions with the DNA in different nucleotide states, providing the structural basis for DNA translocation. Together, our findings suggest a fundamental mechanism for the DNA translocation that underlies chromatin remodelling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6880.map.gz emd_6880.map.gz | 2.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6880-v30.xml emd-6880-v30.xml emd-6880.xml emd-6880.xml | 23.2 KB 23.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_6880.png emd_6880.png | 194.5 KB | ||
| Filedesc metadata |  emd-6880.cif.gz emd-6880.cif.gz | 7.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6880 http://ftp.pdbj.org/pub/emdb/structures/EMD-6880 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6880 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6880 | HTTPS FTP |
-Related structure data
| Related structure data |  5z3oMC  6879C  6882C  6883C  9748C  9749C  5z3lC  5z3uC  5z3vC  6iy2C  6iy3C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6880.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6880.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
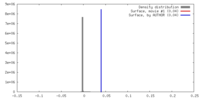
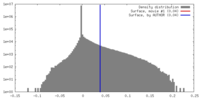
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : complex of snf2 and nucleosome in ADP state
+Supramolecule #1: complex of snf2 and nucleosome in ADP state
+Macromolecule #1: Histone H3.2
+Macromolecule #2: Histone H4
+Macromolecule #3: Histone H2A
+Macromolecule #4: Histone H2B 1.1
+Macromolecule #7: Transcription regulatory protein SNF2
+Macromolecule #5: DNA (167-MER)
+Macromolecule #6: DNA (167-MER)
+Macromolecule #8: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #9: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 1-32 / Number grids imaged: 2 / Number real images: 2388 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 3.1 µm / Calibrated defocus min: 1.7 µm / Calibrated magnification: 22500 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.2 µm / Nominal defocus min: 1.8 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-5z3o: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)