[English] 日本語
 Yorodumi
Yorodumi- EMDB-11312: Structure of the Shigella MxiH needle filament attached to the ba... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11312 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the Shigella MxiH needle filament attached to the basal body | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Type 3 secretion system / Shigella / Helical reconstruction / Needle filament. / PROTEIN TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationtype III protein secretion system complex / protein secretion by the type III secretion system / cell surface / extracellular region / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Shigella flexneri 5a str. M90T (bacteria) Shigella flexneri 5a str. M90T (bacteria) | |||||||||
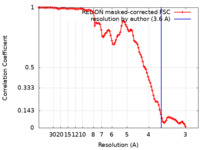
| Method | helical reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Lunelli M / Kotov V | |||||||||
| Funding support |  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Biochem Biophys Rep / Year: 2021 Journal: Biochem Biophys Rep / Year: 2021Title: Helical reconstruction of and needle filaments attached to type 3 basal bodies. Authors: Vadim Kotov / Michele Lunelli / Jiri Wald / Michael Kolbe / Thomas C Marlovits /  Abstract: Gram-negative pathogens evolved a syringe-like nanomachine, termed type 3 secretion system, to deliver protein effectors into the cytoplasm of host cells. An essential component of this system is a ...Gram-negative pathogens evolved a syringe-like nanomachine, termed type 3 secretion system, to deliver protein effectors into the cytoplasm of host cells. An essential component of this system is a long helical needle filament that protrudes from the bacterial surface and connects the cytoplasms of the bacterium and the eukaryotic cell. Previous structural research was predominantly focused on reconstituted type 3 needle filaments, which lacked the biological context. In this work we introduce a facile procedure to obtain high-resolution cryo-EM structure of needle filaments attached to the basal body of type 3 secretion systems. We validate our approach by solving the structure of PrgI filament and demonstrate its utility by obtaining the first high-resolution cryo-EM reconstruction of Shigella MxiH filament. Our work paves the way to systematic structural characterization of attached type 3 needle filaments in the context of mutagenesis studies, protein structural evolution and drug development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11312.map.gz emd_11312.map.gz | 5.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11312-v30.xml emd-11312-v30.xml emd-11312.xml emd-11312.xml | 17.1 KB 17.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_11312_fsc.xml emd_11312_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_11312.png emd_11312.png | 138 KB | ||
| Filedesc metadata |  emd-11312.cif.gz emd-11312.cif.gz | 5.7 KB | ||
| Others |  emd_11312_half_map_1.map.gz emd_11312_half_map_1.map.gz emd_11312_half_map_2.map.gz emd_11312_half_map_2.map.gz | 80.8 MB 81 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11312 http://ftp.pdbj.org/pub/emdb/structures/EMD-11312 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11312 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11312 | HTTPS FTP |
-Validation report
| Summary document |  emd_11312_validation.pdf.gz emd_11312_validation.pdf.gz | 674.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_11312_full_validation.pdf.gz emd_11312_full_validation.pdf.gz | 673.7 KB | Display | |
| Data in XML |  emd_11312_validation.xml.gz emd_11312_validation.xml.gz | 18.3 KB | Display | |
| Data in CIF |  emd_11312_validation.cif.gz emd_11312_validation.cif.gz | 23.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11312 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11312 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11312 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11312 | HTTPS FTP |
-Related structure data
| Related structure data |  6zniMC  6znhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11312.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11312.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.38369 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: #2
| File | emd_11312_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_11312_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Needle filament of the type III secretion system
| Entire | Name: Needle filament of the type III secretion system |
|---|---|
| Components |
|
-Supramolecule #1: Needle filament of the type III secretion system
| Supramolecule | Name: Needle filament of the type III secretion system / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all Details: Structure obtained from needles attached to the basal body |
|---|---|
| Source (natural) | Organism:  Shigella flexneri 5a str. M90T (bacteria) Shigella flexneri 5a str. M90T (bacteria) |
-Macromolecule #1: Protein MxiH
| Macromolecule | Name: Protein MxiH / type: protein_or_peptide / ID: 1 / Number of copies: 23 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Shigella flexneri 5a str. M90T (bacteria) Shigella flexneri 5a str. M90T (bacteria) |
| Molecular weight | Theoretical: 11.060279 KDa |
| Recombinant expression | Organism:  Shigella flexneri 5a str. M90T (bacteria) Shigella flexneri 5a str. M90T (bacteria) |
| Sequence | String: MASWSHPQFE KIEGRMSVTV PNDDWTLSSL SETFDDGTQT LQGELTLALD KLAKNPSNPQ LLAEYQSKLS EYTLYRNAQS NTVKVIKDV DAAIIQNFR UniProtKB: Type 3 secretion system needle filament protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 0.5 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Frames/image: 1-6 / Number grids imaged: 1 / Number real images: 5238 / Average exposure time: 1.5 sec. / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 100000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)