[English] 日本語
 Yorodumi
Yorodumi- EMDB-11268: E2 core of the fungal Pyruvate dehydrogenase complex with flexibl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11268 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | E2 core of the fungal Pyruvate dehydrogenase complex with flexible/oversym. periphery, structured core and S4Y-structured interior. | ||||||||||||||||||
 Map data Map data | Fungal PDC (N. crassa). Endogenous preparation-E1 E2 E3 PX. Enforced symmetry: T. Periphery is flexible/oversym. Core is structured. Interior is structured in arrangement Y / S4Y. | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | acetyl transferase / pyruvate dehydrogenase / protein complex / mitochondria / metabolism / tetrahedral icosahedral / TRANSFERASE | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationdihydrolipoyllysine-residue acetyltransferase / dihydrolipoyllysine-residue acetyltransferase activity / pyruvate decarboxylation to acetyl-CoA / pyruvate dehydrogenase complex / mitochondrial matrix Similarity search - Function | ||||||||||||||||||
| Biological species |  Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) (fungus) Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) (fungus) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.3 Å | ||||||||||||||||||
 Authors Authors | Forsberg BO / Aibara S / Howard RJ / Mortezaei N / Lindahl E | ||||||||||||||||||
| Funding support |  Sweden, European Union, 5 items Sweden, European Union, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Arrangement and symmetry of the fungal E3BP-containing core of the pyruvate dehydrogenase complex. Authors: B O Forsberg / S Aibara / R J Howard / N Mortezaei / E Lindahl /   Abstract: The pyruvate dehydrogenase complex (PDC) is a multienzyme complex central to aerobic respiration, connecting glycolysis to mitochondrial oxidation of pyruvate. Similar to the E3-binding protein (E3BP) ...The pyruvate dehydrogenase complex (PDC) is a multienzyme complex central to aerobic respiration, connecting glycolysis to mitochondrial oxidation of pyruvate. Similar to the E3-binding protein (E3BP) of mammalian PDC, PX selectively recruits E3 to the fungal PDC, but its divergent sequence suggests a distinct structural mechanism. Here, we report reconstructions of PDC from the filamentous fungus Neurospora crassa by cryo-electron microscopy, where we find protein X (PX) interior to the PDC core as opposed to substituting E2 core subunits as in mammals. Steric occlusion limits PX binding, resulting in predominantly tetrahedral symmetry, explaining previous observations in Saccharomyces cerevisiae. The PX-binding site is conserved in (and specific to) fungi, and complements possible C-terminal binding motifs in PX that are absent in mammalian E3BP. Consideration of multiple symmetries thus reveals a differential structural basis for E3BP-like function in fungal PDC. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11268.map.gz emd_11268.map.gz | 73.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11268-v30.xml emd-11268-v30.xml emd-11268.xml emd-11268.xml | 18.5 KB 18.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_11268_fsc.xml emd_11268_fsc.xml | 13 KB | Display |  FSC data file FSC data file |
| Images |  emd_11268.png emd_11268.png | 192.4 KB | ||
| Filedesc metadata |  emd-11268.cif.gz emd-11268.cif.gz | 5.7 KB | ||
| Others |  emd_11268_half_map_1.map.gz emd_11268_half_map_1.map.gz emd_11268_half_map_2.map.gz emd_11268_half_map_2.map.gz | 97.6 MB 97.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11268 http://ftp.pdbj.org/pub/emdb/structures/EMD-11268 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11268 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11268 | HTTPS FTP |
-Related structure data
| Related structure data |  6zlmMC  6zloC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10489 (Title: Native Pyruvate Dehydrogenase Complex from Neurospora crassa EMPIAR-10489 (Title: Native Pyruvate Dehydrogenase Complex from Neurospora crassaData size: 305.5 Data #1: Aligned and dose-weighted micrographs of native N. crassa Pyruvate dehydrogenase [micrographs - single frame]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_11268.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11268.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Fungal PDC (N. crassa). Endogenous preparation-E1 E2 E3 PX. Enforced symmetry: T. Periphery is flexible/oversym. Core is structured. Interior is structured in arrangement Y / S4Y. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.25 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
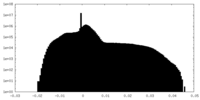
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: Half-map 1. Fungal PDC (N. crassa). Endogenous preparation-E1...
| File | emd_11268_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map 1. Fungal PDC (N. crassa). Endogenous preparation-E1 E2 E3 PX. Enforced symmetry: T. Periphery is flexible/oversym. Core is structured. Interior is structured in arrangement Y / S4Y. | ||||||||||||
| Projections & Slices |
| ||||||||||||
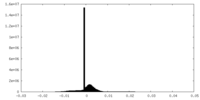
| Density Histograms |
-Half map: Half-map 2. Fungal PDC (N. crassa). Endogenous preparation-E1...
| File | emd_11268_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map 2. Fungal PDC (N. crassa). Endogenous preparation-E1 E2 E3 PX. Enforced symmetry: T. Periphery is flexible/oversym. Core is structured. Interior is structured in arrangement Y / S4Y. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : endogenous pyruvate dehydrogenase complex form Neurospora crassa
| Entire | Name: endogenous pyruvate dehydrogenase complex form Neurospora crassa |
|---|---|
| Components |
|
-Supramolecule #1: endogenous pyruvate dehydrogenase complex form Neurospora crassa
| Supramolecule | Name: endogenous pyruvate dehydrogenase complex form Neurospora crassa type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Catalytic (C-terminal) domain of Dihydrolipoyllysine-residue acetyltransferase (E2-component of pyruvate dehydrogenase complex) |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) (fungus) Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) (fungus)Organelle: mitochondria |
| Molecular weight | Theoretical: 7 MDa |
-Macromolecule #1: Dihydrolipoyllysine-residue acetyltransferase component of pyruva...
| Macromolecule | Name: Dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex, mitochondrial type: protein_or_peptide / ID: 1 / Number of copies: 60 / Enantiomer: LEVO / EC number: dihydrolipoyllysine-residue acetyltransferase |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) (fungus) Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) (fungus) |
| Molecular weight | Theoretical: 48.677395 KDa |
| Sequence | String: MIVPVLSRQA LRHASVARVA LPSLTRWYAS YPPHTVVKMP ALSPTMTSGG IGAWQKKPGD KIEPGEVLVE IETDKAQMDF EFQEEGVLA KILKDSGEKD VAVGNPIAIL VEEGTDVNAF KDFTLKDAGG ETSPAVPKDE PKNESTASAP TPAPTPAPEP E NTSFTGRF ...String: MIVPVLSRQA LRHASVARVA LPSLTRWYAS YPPHTVVKMP ALSPTMTSGG IGAWQKKPGD KIEPGEVLVE IETDKAQMDF EFQEEGVLA KILKDSGEKD VAVGNPIAIL VEEGTDVNAF KDFTLKDAGG ETSPAVPKDE PKNESTASAP TPAPTPAPEP E NTSFTGRF QTALEREPNA LPAAKRLARE KGIDLRNVKG SGPGGKITEE DVKKALASAP AAGAAAAAYT DVPISGMRKT IA ARLKESV TENPHFFVST NLSVSKLLKL RQALNSSADG RYKLSVNDFL IKAMGIASKR VPTVNSSWRD GVIRQFETVD VSV AVATPN GLITPIVKGV EGKGLESISA AVKELAKKAR DGKLKPEEYQ GGSISISNMG MNPAVQSFTA IINPPQAAIL AVGA PQKVA VPVENEDGTT GVSWDEQIIV TASFDHKVVD GAVGAEWIRE LKKVIENPLE LLL UniProtKB: Dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex, mitochondrial |
-Macromolecule #2: Pyruvate dehydrogenase X component
| Macromolecule | Name: Pyruvate dehydrogenase X component / type: protein_or_peptide / ID: 2 / Details: Uniprot Q7RWS2 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) (fungus) Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) (fungus) |
| Molecular weight | Theoretical: 1.124378 KDa |
| Sequence | String: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Detector mode: COUNTING / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


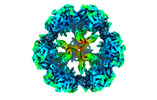










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)