[English] 日本語
 Yorodumi
Yorodumi- EMDB-10951: Condensin complex from S.cerevisiae ATP-free apo non-engaged stat... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10951 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Condensin complex from S.cerevisiae ATP-free apo non-engaged state: overall map | ||||||||||||
 Map data Map data | overall | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | essential for the functional organization of genomes / CELL CYCLE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of meiotic DNA double-strand break formation / Condensation of Prometaphase Chromosomes / tRNA gene clustering / meiotic chromosome condensation / meiotic chromosome separation / condensin complex / DNA secondary structure binding / maintenance of rDNA / rDNA chromatin condensation / nucleophagy ...negative regulation of meiotic DNA double-strand break formation / Condensation of Prometaphase Chromosomes / tRNA gene clustering / meiotic chromosome condensation / meiotic chromosome separation / condensin complex / DNA secondary structure binding / maintenance of rDNA / rDNA chromatin condensation / nucleophagy / synaptonemal complex assembly / condensed chromosome, centromeric region / mitotic chromosome condensation / chromosome condensation / silent mating-type cassette heterochromatin formation / minor groove of adenine-thymine-rich DNA binding / mitotic sister chromatid segregation / condensed chromosome / double-stranded DNA binding / histone binding / cell division / chromatin binding / chromatin / nucleolus / ATP hydrolysis activity / mitochondrion / ATP binding / nucleus / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 7.5 Å | ||||||||||||
 Authors Authors | Lee B-G / Cawood C / Gutierrez-Escribano P / Nakane T / Merkel F / Hassler M / Haering CH / Aragon L / Lowe J | ||||||||||||
| Funding support |  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2020 Journal: Nat Struct Mol Biol / Year: 2020Title: Cryo-EM structures of holo condensin reveal a subunit flip-flop mechanism. Authors: Byung-Gil Lee / Fabian Merkel / Matteo Allegretti / Markus Hassler / Christopher Cawood / Léa Lecomte / Francis J O'Reilly / Ludwig R Sinn / Pilar Gutierrez-Escribano / Marc Kschonsak / Sol ...Authors: Byung-Gil Lee / Fabian Merkel / Matteo Allegretti / Markus Hassler / Christopher Cawood / Léa Lecomte / Francis J O'Reilly / Ludwig R Sinn / Pilar Gutierrez-Escribano / Marc Kschonsak / Sol Bravo / Takanori Nakane / Juri Rappsilber / Luis Aragon / Martin Beck / Jan Löwe / Christian H Haering /    Abstract: Complexes containing a pair of structural maintenance of chromosomes (SMC) family proteins are fundamental for the three-dimensional (3D) organization of genomes in all domains of life. The ...Complexes containing a pair of structural maintenance of chromosomes (SMC) family proteins are fundamental for the three-dimensional (3D) organization of genomes in all domains of life. The eukaryotic SMC complexes cohesin and condensin are thought to fold interphase and mitotic chromosomes, respectively, into large loop domains, although the underlying molecular mechanisms have remained unknown. We used cryo-EM to investigate the nucleotide-driven reaction cycle of condensin from the budding yeast Saccharomyces cerevisiae. Our structures of the five-subunit condensin holo complex at different functional stages suggest that ATP binding induces the transition of the SMC coiled coils from a folded-rod conformation into a more open architecture. ATP binding simultaneously triggers the exchange of the two HEAT-repeat subunits bound to the SMC ATPase head domains. We propose that these steps result in the interconversion of DNA-binding sites in the catalytic core of condensin, forming the basis of the DNA translocation and loop-extrusion activities. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10951.map.gz emd_10951.map.gz | 188.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10951-v30.xml emd-10951-v30.xml emd-10951.xml emd-10951.xml | 28 KB 28 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_10951.png emd_10951.png | 56.9 KB | ||
| Filedesc metadata |  emd-10951.cif.gz emd-10951.cif.gz | 9.8 KB | ||
| Others |  emd_10951_additional_1.map.gz emd_10951_additional_1.map.gz emd_10951_additional_2.map.gz emd_10951_additional_2.map.gz | 83.7 MB 13.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10951 http://ftp.pdbj.org/pub/emdb/structures/EMD-10951 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10951 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10951 | HTTPS FTP |
-Related structure data
| Related structure data |  6yvuMC  6yvdC  6yvvC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10951.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10951.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | overall | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Supplemental map: emd 10951 additional 1.map
| File | emd_10951_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
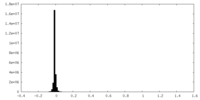
| Density Histograms |
-Supplemental map: emd 10951 additional 2.map
| File | emd_10951_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
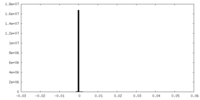
| Density Histograms |
- Sample components
Sample components
-Entire : Condensin
| Entire | Name: Condensin |
|---|---|
| Components |
|
-Supramolecule #1: Condensin
| Supramolecule | Name: Condensin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: complex of 5 protein subunits: Smc2; Smc4; Brn1; Ycs4; Ycg1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 500 MDa |
-Macromolecule #1: Structural maintenance of chromosomes protein 2,Structural mainte...
| Macromolecule | Name: Structural maintenance of chromosomes protein 2,Structural maintenance of chromosomes protein 2,Smc2 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 134.80675 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKVEELIIDG FKSYATRTVI TDWDPQFNAI TGLNGSGKSN ILDAICFVLG IASMSTVRAS SLQDLIYKRG QAGVTKASVT IVFDNTDKS NSPIGFTNSP QISVTRQVVL GGTSKYLING HRAPQQSVLQ LFQSVQLNIN NPNFLIMQGK ITKVLNMKPS E ILSLIEEA ...String: MKVEELIIDG FKSYATRTVI TDWDPQFNAI TGLNGSGKSN ILDAICFVLG IASMSTVRAS SLQDLIYKRG QAGVTKASVT IVFDNTDKS NSPIGFTNSP QISVTRQVVL GGTSKYLING HRAPQQSVLQ LFQSVQLNIN NPNFLIMQGK ITKVLNMKPS E ILSLIEEA AGTKMFEDRR EKAERTMSKK ETKLQENRTL LTEEIEPKLE KLRNEKRMFL EFQSTQTDLE KTERIVVSYE YY NIKHKHT SIRETLENGE TRMKMLNEFV KKTSEEIDSL NEDVEEIKLQ KEKELHKEGT ISKLENKENG LLNEISRLKT SLS IKVENL NDTTEKSKAL ESEIASSSAK LIEKKSAYAN TEKDYKMVQE QLSKQRDLYK RKEELVSTLT TGISSTGAAD GGYN AQLAK AKTELNEVSL AIKKSSMKME LLKKELLTIE PKLKEATKDN ELNVKHVKQC QETCDKLRAR LVEYGFDPSR IKDLK QRED KLKSHYYQTC KNSEYLKRRV TNLEFNYTKP YPNFEASFVH GVVGQLFQID NDNIRYATAL QTCAGGRLFN VVVQDS QTA TQLLERGRLR KRVTIIPLDK IYTRPISSQV LDLAKKIAPG KVELAINLIR FDESITKAME FIFGNSLICE DPETAKK IT FHPKIRARSI TLQGDVYDPE GTLSGGSRNT SESLLVDIQK YNQIQKQIET IQADLNHVTE ELQTQYATSQ KTKTIQSD L NLSLHKLDLA KRNLDANPSS QIIARNEEIL RDIGECENEI KTKQMSLKKC QEEVSTIEKD MKEYDSDKGS KLNELKKEL KLLAKELEEQ ESESERKYDL FQNLELETEQ LSSELDSNKT LLHNHLKSIE SLKLENSDLE GKIRGVEDDL VTVQTELNEE KKRLMDIDD ELNELETLIK KKQDEKKSSE LELQKLVHDL NKYKSNTNNM EKIIEDLRQK HEFLEDFDLV RNIVKQNEGI D LDTYRERS KQLNEKFQEL RKKVNPNIMN MIENVEKKEA ALKTMIKTIE KDKMKIQETI SKLNEYKRET LVKTWEKVTL DF GNIFADL LPNSFAKLVP CEGKDVTQGL EVKVKLGNIW KESLIELSGG QRSLIALSLI MALLQFRPAP MYILDEVDAA LDL SHTQNI GHLIKTRFKG SQFIVVSLKE GMFANANRVF RTRFQDGTSV VSIM(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) UniProtKB: Structural maintenance of chromosomes protein 2 |
-Macromolecule #2: Structural maintenance of chromosomes protein 4
| Macromolecule | Name: Structural maintenance of chromosomes protein 4 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 162.435812 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSDSPLSKRQ KRKSAQEPEL SLDQGDAEED SQVENRVNLS ENTPEPDLPA LEASYSKSYT PRKLVLSSGE NRYAFSQPTN STTTSLHVP NLQPPKTSSR GRDHKSYSQS PPRSPGRSPT RRLELLQLSP VKNSRVELQK IYDRHQSSSK QQSRLFINEL V LENFKSYA ...String: MSDSPLSKRQ KRKSAQEPEL SLDQGDAEED SQVENRVNLS ENTPEPDLPA LEASYSKSYT PRKLVLSSGE NRYAFSQPTN STTTSLHVP NLQPPKTSSR GRDHKSYSQS PPRSPGRSPT RRLELLQLSP VKNSRVELQK IYDRHQSSSK QQSRLFINEL V LENFKSYA GKQVVGPFHT SFSAVVGPNG SGKSNVIDSM LFVFGFRANK MRQDRLSDLI HKSEAFPSLQ SCSVAVHFQY VI DESSGTS RIDEEKPGLI ITRKAFKNNS SKYYINEKES SYTEVTKLLK NEGIDLDHKR FLILQGEVEN IAQMKPKAEK ESD DGLLEY LEDIIGTANY KPLIEERMGQ IENLNEVCLE KENRFEIVDR EKNSLESGKE TALEFLEKEK QLTLLRSKLF QFKL LQSNS KLASTLEKIS SSNKDLEDEK MKFQESLKKV DEIKAQRKEI KDRISSCSSK EKTLVLERRE LEGTRVSLEE RTKNL VSKM EKAEKTLKST KHSISEAENM LEELRGQQTE HETEIKDLTQ LLEKERSILD DIKLSLKDKT KNISAEIIRH EKELEP WDL QLQEKESQIQ LAESELSLLE ETQAKLKKNV ETLEEKILAK KTHKQELQDL ILDLKKKLNS LKDERSQGEK NFTSAHL KL KEMQKVLNAH RQRAMEARSS LSKAQNKSKV LTALSRLQKS GRINGFHGRL GDLGVIDDSF DVAISTACPR LDDVVVDT V ECAQHCIDYL RKNKLGYARF ILLDRLRQFN LQPISTPENV PRLFDLVKPK NPKFSNAFYS VLRDTLVAQN LKQANNVAY GKKRFRVVTV DGKLIDISGT MSGGGNHVAK GLMKLGTNQS DKVDDYTPEE VDKIERELSE RENNFRVASD TVHEMEEELK KLRDHEPDL ESQISKAEME ADSLASELTL AEQQVKEAEM AYVKAVSDKA QLNVVMKNLE RLRGEYNDLQ SETKTKKEKI K GLQDEIMK IGGIKLQMQN SKVESVCQKL DILVAKLKKV KSASKKSGGD VVKFQKLLQN SERDVELSSD ELKVIEEQLK HT KLALAEN DTNMNETLNL KVELKEQSEQ LKEQMEDMEE SINEFKSIEI EMKNKLEKLN SLLTYIKSEI TQQEKGLNEL SIR DVTHTL GMLDDNKMDS VKEDVKNNQE LDQEYRSCET QDESEIKDAE TSCDNYHPMN IDETSDEVSR GIPRLSEDEL RELD VELIE SKINELSYYV EETNVDIGVL EEYARRLAEF KRRKLDLNNA VQKRDEVKEQ LGILKKKRFD EFMAGFNIIS MTLKE MYQM ITMGGNAELE LVDSLDPFSE GVTFSVMPPK KSWRNITNLS GGEKTLSSLA LVFALHKYKP TPLYVMDEID AALDFR NVS IVANYIKERT KNAQFIVISL RNNMFELAQQ LVGVYKRDNR TKSTTIKNID ILNRT UniProtKB: Structural maintenance of chromosomes protein 4 |
-Macromolecule #3: Condensin complex subunit 2,Condensin complex subunit 2,Brn1
| Macromolecule | Name: Condensin complex subunit 2,Condensin complex subunit 2,Brn1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 87.940234 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTTQLRYENN DDDERVEYNL FTNRSTMMAN FEEWIKMATD NKINSRNSWN FALIDYFYDL DVLKDGENNI NFQKASATLD GCIKIYSSR VDSVTTETGK LLSGLAQRKT NGASNGDDSN GGNGEGLGGD SDEANIEIDP LTGMPISNDP DVNNTRRRVY N RVLETTLV ...String: MTTQLRYENN DDDERVEYNL FTNRSTMMAN FEEWIKMATD NKINSRNSWN FALIDYFYDL DVLKDGENNI NFQKASATLD GCIKIYSSR VDSVTTETGK LLSGLAQRKT NGASNGDDSN GGNGEGLGGD SDEANIEIDP LTGMPISNDP DVNNTRRRVY N RVLETTLV EFETIKMKEL DQELIIDPLF KKALVDFDEG GAKSLLLNTL NIDNTARVIF DASIKDTQNV GQGKLQRKEE EL IERDSLV DDENEPSQSL ISTRNDSTVN DSVISAPSME DEILSLGMDF IKFDQIAVCE ISGSIEQLRN VVEDINQAKD FIE NVNNRF DNFLTEEELQ AAVPDNAEDD SDGFDMGMQQ ELCYPDENHD NTSHDEQDDD NVNSTTGSIF EKDLMAYFDE NLNR NWRGR EHWKVRNFKK ANLVNKESDL LEETRTTIGD TTDKNTTDDK SMDTKKKHKQ KKVLEIDFFK TDDSFEDKVF ASKGR TKID MPIKNRKNDT HYLLPDDFHF STDRITRLFI KPGQKMSLFS HRKHTRGDVS SGLFEKSTVS ANHSNNDIPT IADEHF WAD NYERKEQEEK EKEQSKEVGD VVGGALDNPF EDDMDGVDFN QAFEGTDDNE EASVKLDLQD DEDHKFPIRE NKVTYSR VS KKVDVRRLKK NVWRSINNLI QEHDSRKNRE QSSNDSETHT EDESTKELKF SDIIQGISKM YSDDTLKDIS TSFCFICL L HLANEHGLQI THTENYNDLI VNYEDLATTQ AAS(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) UniProtKB: Condensin complex subunit 2 |
-Macromolecule #4: Condensin complex subunit 1,Condensin complex subunit 1,Ycs4
| Macromolecule | Name: Condensin complex subunit 1,Condensin complex subunit 1,Ycs4 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 133.88275 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGFSLSEYL TKFQTTDRES YPRLQDPSRE LNVIIDQLAV SPEQIDASPD SLEALIDLCH DFPHLTPKLQ TQLSYLISSS LSNLSKDIK ANLSSNVNFT EIGGLIPQWK RHLEEYGYLI QVLLTFLQDE LHKVSSQSTN LNRSAKNSKN DSANVELFKR D CNQMENLL ...String: MSGFSLSEYL TKFQTTDRES YPRLQDPSRE LNVIIDQLAV SPEQIDASPD SLEALIDLCH DFPHLTPKLQ TQLSYLISSS LSNLSKDIK ANLSSNVNFT EIGGLIPQWK RHLEEYGYLI QVLLTFLQDE LHKVSSQSTN LNRSAKNSKN DSANVELFKR D CNQMENLL ESITKLLEIN LSKIFQTTPE KDLFIGLFTR PLFVLLEIEP VTKVSSLKMF IQRILAMCVK NHGQSSSIQS SL MTNLTYF LHLSVFNAEL LKLLNDEYNY PQLTEDILKE ISTRVFNAKD TTGPKAISNF LIKLSELSPG IMLRQMNLVI TLL NNSSIT LRCSVVEACG NIVAELAQDP QTMEHYKQQI AVLIELLEER FQDSNPYVRT KAIQGCSKIC DLSSKFNKSK AKFT SLAVR SLQDRSSLVR RNSVKLLSKL LLKHPFKAIH GSQLRLSEWE EYLKGSESQL NSTLKKVESQ ETLNDTIERS LIEEE VEQD EGQCRTELEG SFNKSAELSR IENEVENINA TNTSVLMKLK LMIVYYKDAI SFIKEIHKSI ELISNLLFSK NRNEVL ESM DFLVLADAFD IELSEFGIKK MLHLVWMKGT NDEGTSISVH LIECYKQLFL TAPDSCNMQE KAAHIAKNLI NLSIGAS IA DLASLEQLLG MMYEQKLIDQ HVINILWAIY NSASKASMQK EQNVNNRDSE KGFSKEQIHG SIIILGMLSL ADNEIALK G LESLLNIGLG AVGLKDLTLC RYSCLALERM VPKRSTIITK AINQELEDVA VKKLYAIIIN YTKDNEYYPM CEQALSALF TISSKPDILA TDLIREKTMM TFGKPEEEDS ILSLEQSSRV VSLSQLLFIV GQVAIKTLVY LEKCEAEFKK RKIEAETRNG KVKNQGADV TNTTQDNGGD KELEMIGGTN EDDFTDAIQF VKENELLFGE KSILGKFCPI VEEIVSNSSR FSDPMLQRTA T LCLEKLMC LSSKYCEKSL PLLITVMEKS PDPTIRSNAV LGLGDMAVCF NNLVDENTDY LYRRLHDENL MVQRTCLMTV TF LILAGQV KVKGQLGEMA KCLDNPDQGI SDMCRLFFTE LASKDNAIYN GFIDIFSNLS SDDLLGKESF KKIIKFLLTF IDK ERHQKQ LNEKLVGRLR KCETQKQWDD IAFVLNNLPY KNEDVTALLE QGFKVVSAKE (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) UniProtKB: Condensin complex subunit 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Component - Name: Tris |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 100 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE / Energy filter - Slit width: 20 eV |
| Image recording | #0 - Image recording ID: 1 / #0 - Film or detector model: GATAN K2 SUMMIT (4k x 4k) / #0 - Detector mode: COUNTING / #0 - Number grids imaged: 1 / #0 - Number real images: 10000 / #0 - Average exposure time: 10.0 sec. / #0 - Average electron dose: 45.0 e/Å2 / #1 - Image recording ID: 2 / #1 - Film or detector model: GATAN K2 SUMMIT (4k x 4k) / #1 - Detector mode: COUNTING / #1 - Average electron dose: 1.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||||||||||||
| Output model |  PDB-6yvu: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)