登録情報 データベース : EMDB / ID : EMD-10496タイトル Cryo-EM Structure of as isolated form of NAD+-dependent Formate Dehydrogenase from Rhodobacter capsulatus 複合体 : Formate dehydrogenase, as isolatedタンパク質・ペプチド : Formate dehydrogenase subunit alphaタンパク質・ペプチド : Formate dehydrogenase subunit betaタンパク質・ペプチド : Formate dehydrogenase subunit gammaタンパク質・ペプチド : NAD-dependent formate dehydrogenase subunit deltaリガンド : 2-AMINO-5,6-DIMERCAPTO-7-METHYL-3,7,8A,9-TETRAHYDRO-8-OXA-1,3,9,10-TETRAAZA-ANTHRACEN-4-ONE GUANOSINE DINUCLEOTIDEリガンド : MOLYBDENUM(VI) IONリガンド : FE2/S2 (INORGANIC) CLUSTERリガンド : IRON/SULFUR CLUSTERリガンド : HYDROSULFURIC ACIDリガンド : FLAVIN MONONUCLEOTIDE / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
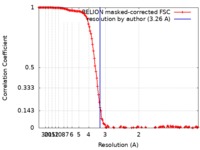
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Rhodobacter capsulatus (バクテリア)手法 / / 解像度 : 3.26 Å Wendler P / Radon C 資金援助 Organization Grant number 国 German Research Foundation EXC 314/2 German Research Foundation EXC 2008/1 European Union iNext-4008
ジャーナル : Nat Commun / 年 : 2020タイトル : Cryo-EM structures reveal intricate Fe-S cluster arrangement and charging in Rhodobacter capsulatus formate dehydrogenase.著者 : Christin Radon / Gerd Mittelstädt / Benjamin R Duffus / Jörg Bürger / Tobias Hartmann / Thorsten Mielke / Christian Teutloff / Silke Leimkühler / Petra Wendler / 要旨 : Metal-containing formate dehydrogenases (FDH) catalyse the reversible oxidation of formate to carbon dioxide at their molybdenum or tungsten active site. They display a diverse subunit and cofactor ... Metal-containing formate dehydrogenases (FDH) catalyse the reversible oxidation of formate to carbon dioxide at their molybdenum or tungsten active site. They display a diverse subunit and cofactor composition, but structural information on these enzymes is limited. Here we report the cryo-electron microscopic structures of the soluble Rhodobacter capsulatus FDH (RcFDH) as isolated and in the presence of reduced nicotinamide adenine dinucleotide (NADH). RcFDH assembles into a 360 kDa dimer of heterotetramers revealing a putative interconnection of electron pathway chains. In the presence of NADH, the RcFDH structure shows charging of cofactors, indicative of an increased electron load. 履歴 登録 2019年11月15日 - ヘッダ(付随情報) 公開 2020年4月22日 - マップ公開 2020年4月22日 - 更新 2024年5月22日 - 現状 2024年5月22日 処理サイト : PDBe / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Rhodobacter capsulatus (バクテリア)
Rhodobacter capsulatus (バクテリア) データ登録者
データ登録者 ドイツ, 3件
ドイツ, 3件  引用
引用 ジャーナル: Nat Commun / 年: 2020
ジャーナル: Nat Commun / 年: 2020

 構造の表示
構造の表示 ムービービューア
ムービービューア SurfView
SurfView Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク emd_10496.map.gz
emd_10496.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-10496-v30.xml
emd-10496-v30.xml emd-10496.xml
emd-10496.xml EMDBヘッダ
EMDBヘッダ emd_10496_fsc.xml
emd_10496_fsc.xml FSCデータファイル
FSCデータファイル emd_10496.png
emd_10496.png emd-10496.cif.gz
emd-10496.cif.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-10496
http://ftp.pdbj.org/pub/emdb/structures/EMD-10496 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10496
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10496 emd_10496_validation.pdf.gz
emd_10496_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_10496_full_validation.pdf.gz
emd_10496_full_validation.pdf.gz emd_10496_validation.xml.gz
emd_10496_validation.xml.gz emd_10496_validation.cif.gz
emd_10496_validation.cif.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10496
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10496 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10496
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10496 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_10496.map.gz / 形式: CCP4 / 大きさ: 216 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_10496.map.gz / 形式: CCP4 / 大きさ: 216 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 画像解析
画像解析
 ムービー
ムービー コントローラー
コントローラー

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)