[English] 日本語
 Yorodumi
Yorodumi- PDB-6tg9: Cryo-EM Structure of NADH reduced form of NAD+-dependent Formate ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6tg9 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structure of NADH reduced form of NAD+-dependent Formate Dehydrogenase from Rhodobacter capsulatus | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | OXIDOREDUCTASE / molybdoenzyme / formate oxidation / NAD+-dependent | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationformate dehydrogenase complex / formate metabolic process / formate dehydrogenase / formate dehydrogenase / formate dehydrogenase (NAD+) activity / oxidoreductase complex / molybdopterin cofactor binding / NADH dehydrogenase activity / NADH dehydrogenase (ubiquinone) activity / respiratory electron transport chain ...formate dehydrogenase complex / formate metabolic process / formate dehydrogenase / formate dehydrogenase / formate dehydrogenase (NAD+) activity / oxidoreductase complex / molybdopterin cofactor binding / NADH dehydrogenase activity / NADH dehydrogenase (ubiquinone) activity / respiratory electron transport chain / 2 iron, 2 sulfur cluster binding / FMN binding / 4 iron, 4 sulfur cluster binding / oxidoreductase activity / electron transfer activity / metal ion binding / membrane Similarity search - Function | ||||||||||||
| Biological species |  Rhodobacter capsulatus (bacteria) Rhodobacter capsulatus (bacteria) | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.24 Å | ||||||||||||
 Authors Authors | Wendler, P. / Radon, C. / Mittelstaedt, G. | ||||||||||||
| Funding support |  Germany, 3items Germany, 3items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Cryo-EM structures reveal intricate Fe-S cluster arrangement and charging in Rhodobacter capsulatus formate dehydrogenase. Authors: Christin Radon / Gerd Mittelstädt / Benjamin R Duffus / Jörg Bürger / Tobias Hartmann / Thorsten Mielke / Christian Teutloff / Silke Leimkühler / Petra Wendler /   Abstract: Metal-containing formate dehydrogenases (FDH) catalyse the reversible oxidation of formate to carbon dioxide at their molybdenum or tungsten active site. They display a diverse subunit and cofactor ...Metal-containing formate dehydrogenases (FDH) catalyse the reversible oxidation of formate to carbon dioxide at their molybdenum or tungsten active site. They display a diverse subunit and cofactor composition, but structural information on these enzymes is limited. Here we report the cryo-electron microscopic structures of the soluble Rhodobacter capsulatus FDH (RcFDH) as isolated and in the presence of reduced nicotinamide adenine dinucleotide (NADH). RcFDH assembles into a 360 kDa dimer of heterotetramers revealing a putative interconnection of electron pathway chains. In the presence of NADH, the RcFDH structure shows charging of cofactors, indicative of an increased electron load. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6tg9.cif.gz 6tg9.cif.gz | 571 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6tg9.ent.gz pdb6tg9.ent.gz | 460.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6tg9.json.gz 6tg9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tg/6tg9 https://data.pdbj.org/pub/pdb/validation_reports/tg/6tg9 ftp://data.pdbj.org/pub/pdb/validation_reports/tg/6tg9 ftp://data.pdbj.org/pub/pdb/validation_reports/tg/6tg9 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  10495MC  6tgaC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Formate dehydrogenase subunit ... , 3 types, 6 molecules AEBFGC
| #1: Protein | Mass: 104589.211 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Rhodobacter capsulatus (bacteria) / Gene: U715_16550 / Production host: Rhodobacter capsulatus (bacteria) / Gene: U715_16550 / Production host:  Rhodobacter capsulatus (bacteria) / References: UniProt: A0A0E2PAE3, UniProt: D5AQH0*PLUS Rhodobacter capsulatus (bacteria) / References: UniProt: A0A0E2PAE3, UniProt: D5AQH0*PLUS#2: Protein | Mass: 52755.770 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Rhodobacter capsulatus (bacteria) / Gene: U715_16555 / Production host: Rhodobacter capsulatus (bacteria) / Gene: U715_16555 / Production host:  Rhodobacter capsulatus (bacteria) / References: UniProt: A0A0E2P9P2, UniProt: D5AQH1*PLUS Rhodobacter capsulatus (bacteria) / References: UniProt: A0A0E2P9P2, UniProt: D5AQH1*PLUS#3: Protein | Mass: 15603.050 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Rhodobacter capsulatus (bacteria) / Gene: U715_16560 / Production host: Rhodobacter capsulatus (bacteria) / Gene: U715_16560 / Production host:  Rhodobacter capsulatus (bacteria) Rhodobacter capsulatus (bacteria)References: UniProt: A0A0E2PAI9, UniProt: D5AQH2*PLUS, formate dehydrogenase |
|---|
-Protein , 1 types, 2 molecules DH
| #4: Protein | Mass: 7388.531 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Rhodobacter capsulatus (bacteria) / Gene: U715_16540 / Production host: Rhodobacter capsulatus (bacteria) / Gene: U715_16540 / Production host:  Rhodobacter capsulatus (bacteria) / References: UniProt: A0A0E2P9Z0, UniProt: D5AQG8*PLUS Rhodobacter capsulatus (bacteria) / References: UniProt: A0A0E2P9Z0, UniProt: D5AQG8*PLUS |
|---|
-Non-polymers , 7 types, 26 molecules 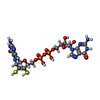










| #5: Chemical | ChemComp-MGD / #6: Chemical | #7: Chemical | ChemComp-FES / #8: Chemical | ChemComp-SF4 / #9: Chemical | #10: Chemical | #11: Chemical | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Formate dehydrogenase, NADH reduced / Type: COMPLEX / Entity ID: #1-#4 / Source: RECOMBINANT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.36 MDa / Experimental value: YES | ||||||||||||
| Source (natural) | Organism:  Rhodobacter capsulatus (bacteria) Rhodobacter capsulatus (bacteria) | ||||||||||||
| Source (recombinant) | Organism:  Rhodobacter capsulatus (bacteria) Rhodobacter capsulatus (bacteria) | ||||||||||||
| Buffer solution | pH: 7.5 | ||||||||||||
| Buffer component |
| ||||||||||||
| Specimen | Conc.: 0.1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R2/4 | ||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company /  Model: Titan Krios / Image courtesy: FEI Company | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM imaging | Accelerating voltage: 300 kV / Electron source:
| |||||||||||||||
| Image recording |
|
- Processing
Processing
| Software | Name: PHENIX / Version: 1.15.2_3472: / Classification: refinement | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||
| CTF correction | Details: CTFFIND4 was used to estimate contrast transfer function parameters. CTF correction was done in Relion 3.0. Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C2 (2 fold cyclic) | ||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.24 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 199229 / Symmetry type: POINT | ||||||||||||||||||||||||||||
| Atomic model building | Protocol: BACKBONE TRACE / Space: REAL | ||||||||||||||||||||||||||||
| Atomic model building |
| ||||||||||||||||||||||||||||
| Refinement | Highest resolution: 3.24 Å | ||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera






 PDBj
PDBj

























